Cloning bovine beta casein, Fam20C (Kex + & -), from 27Sep2014
<html>
Cloning bovine beta casein Band Fam20C Kex +/- gBlocks into pD1214, transforming into E. coli and extracting plasmid DNA
Overview
During this series of experiments, we will insert three gene constructs, below, into the pD1214 vector and transform our constructs into E. coli. We will then perform midiprep plasmid DNA extractions, quantify our plasmid DNA, and submit all three plasmid constructs for sequencing. Sequence-confirmed DNA is then ready to be transformed into yeast.
Genes to be cloned & pD1214 vector
8) P.FAKS.bovBeta(B).S = bovine beta casein B
9) Sap.Faks.hFam20C(Kex+).Sap = this is the gene for the FAM20C kinase involved in phosphorylating secreted proteins, including caseins. The DNA sequence of our "Kex+" variant translates to the native amino acid sequence, including the recognition sequences for the Kex protease.
10) Sap.Faks.hFam20C(Kex-).Sap = this is the gene for the FAM20C kinase involved in phosphorylating secreted proteins, including caseins. In case the Kex protease cleaves this protein before activity, our "Kex-" variant eliminates amino acid recognition sequences for the Kex protease by replacing with biochemically similar amino acids.
These Integrated DNA Technologies (IDT) gBlock sequences have 5' and 3' SapI sequences, followed by full length FAKS, followed by the gene of interest, codon-optimized for expression in S. cerevisiae. Electra cloning cleaves at SapI sites and ligates simultaneously.
We will clone into pD1214. This vector was ordered from DNA2.0 and is linearized upon arrival. Our insert is treated with Electra enzyme mix which cuts and ligates our "SapI.gene.SapI" and inserts it into the pD1214 vector.
SapI treated DNA has an ATG overhang at the 5' end and a GGT overhang at the 3' end. Our complete vector will have the alphafactorfull signal sequence, which leads into another Methionine (M), followed by the rest of the human kappa casein protein.
All you need to know about the Electra system.
Cloning casein & FAM20C gBlocks into pD1214
September 27, 2014
Participants: Maria, Gabby, Rebecca, Johan, Meenakshi, Rachel, Lafia, Nikola
Notes: Rachel
Location: BioCurious
Materials & Methods, Electra Cloning:
We resuspended all lyophilized IDT gBlock DNA as in DNA Handling using 0.5X TE (1X TE, pH 7.5: 1 diH2O). Final DNA concentration = 20ng/uL, per Electra cloning kit requirements.
Used DNA 2.0 Electra cloning kit protocol.
Reaction:
1uL pD1214 (20ng)
1uL of appropriate casein gBlock or (+) control DNA (20ng), or 0.5X TE (-) control
2uL Electra Buffer
1uL of Electra enzyme mix
15uL of diH20
----
20uL
As possible that multiple pipetting stages contributed to failed cloning reaction for P.FAKS.humBeta.S 12Sep2014 (Cloning 7 casein gBlocks, 12Sep2014 and on), we're making a master mix that includes all reagents common to each reaction. Adding 5.5X each reagent for 5 reactions and 0.5 extra.
Master mix (MUST BE MIXED THOROUGHLY) and cloning reactions:
5.5uL pD1214 (20ng/uL)
11.0uL Electra Buffer
5.5uL of Electra enzyme mix
82.5uL of diH20
----
19uL of master mix to each reaction tube + 1uL appropriate gBlock DNA (20ng/uL) or control.
20 min at room temperature.
Completed 5 separate reactions, one for each gBlock, one positive control using DNA supplied in the kit, one negative control with 0.5XTE resuspension buffer in place of DNA insert.
5uL reaction products used immediate for transformation into E. coli, below. Remainder stored at -20°C, BioCurious freezer. Each tube labeled with date and construct ID.
Transforming bovine beta casein B & FAM20C gBlocks:pD1214 cloning reaction products into E. coli
September 27, 2014
Participants: Maria, Gabby, Rebecca, Johan, Meenakshi, Rachel, Lafia, Nikola
Notes: Rachel
Location: BioCurious
Materials & Methods, Transforming Mix & Go competent dH5alpha E. coli with gBlocks in pD1214, above:
We used the Zymo Research instruction manual for "Premade Mix & Go Competent E. coli Cells." Media:Zymo_E._coli_competent_cells_transformation_protocol.pdf
We completed 7 reactions: one for each of the 5 Electra cloning reactions, one pGLO positive control, one competent cells only negative control.
Thawed individual tubes of Mix & Go chemically competent, dH5alpha E. coli cells slowly on ice.
Prewarmed LB agar plates with Carb-50, 100mm (Teknova), at 37°C for until temperature read >34°C with temperature scanner: 1 hr 10 min.
Inducible promoter for pGLO positive control requires arabinose, so used a plate that already contained [need concentration] of arabinose for that reaction.
Added 5uL of each casein:pD1214 or appropriate control to individual thawed tube of 100uL competent cells. Mixed gently.
Immediately incubated on ice for 30 min.
In UV- & 70% isopropanol-sterilized laminar flow hood, plated 105uL of each reaction to LB carb plates, using one individually wrapped spreader/reaction.
Incubated upside down plates overnight at 37°C.
September 28, 2014
Results
The three experimental plates (Bovine Beta B, FAM20C Kex +, FAM20C Kex- in pD1214), Electra cloning positive control and PGlo transformation positive control had >1000 colonies each.
The transformation negative control plate had no colonies.
However, the Electra cloning negative control (control that included all Electra cloning reagents and the TE buffer in which we resuspended our IDT gBlocks; excluding only IDT gBlock "mother vector" DNA), had 46 colonies. Problem!
Discussion and Future Directions
Troubleshooting failed negative control: As transformation negative control plate was blank, contamination unlikely to have occurred while plating in flow hood or in competent cells. Colonies also unlikely due to non-functional antibiotics in LB plates. Some possibilities: 1) contamination of Electra reagents or protocol equipment (pipettes, etc.) by plasmid conferring amp-resistance (one of our plasmids or another AmpR plasmid somehow ambient in lab) or 2) contamination of same by AmpR E. coli cells. 3) Perhaps the linearized pD1214 plasmid can self-ligate at some low frequency, despite incompatible overhangs.
We will plate 1uL of each Electra reagent and the 0.5X TE resuspension buffer (27Sep2014 cloning, above) brought to 100uL in our diH20 on an LB carb plate to see if resistant E. coli colonies grow.
We will perform a plasmid midiprep DNA extraction on a liquid culture of one of the colonies from the Electra negative control plate for sequencing and potential further characterization. This will allow us to tell whether this colony contains self-ligated pD1214 or one of our insert-containing plasmids.
As the experimental plates grew so many more colonies than the failed Electra negative control, many of the colonies likely grew from a cell containing our target plasmid. We will also inoculate liquid cultures from 3 colonies per plate for plasmid DNA extraction and DNA sequencing.
Plasmid DNA midipreps of bovine Beta casein B, FAM20C Kex + & Kex -(in pD1214) and Electra cloning negative control
September 28, 2014
Participants: Johan
Location: BioCurious
Aims: Inoculate overnight cultures of transformation plates from 27Sep2014 reactions for plasmid prep on 29Sep2014.
Materials and Methods:
Inoculated three colonies each from 27Sep2014 transformation reaction plates for bovine Beta casein B, FAM20C Kex + & Kex - in pD1214 into 6mL LB carbenicillin [need carb concentration] liquid medium. Chose three colonies due to potential contamination highlighted by Electra cloning negative control failure (see Results & Discussion, 28Sep2014).
Also inoculated two colonies from Electra cloning negative plate for further troubleshooting to LB carb.
Incubated 11 cultures in 37°C shaking incubator. Cultures started at 10pm.
September 29, 2014
Participants: Lafia, Aaron, Johan, Meenakshi, Advait, Rachel, Nikola, Matt
- Notes: Rachel
- Location: BioCurious
- Aims: Plasmid midipreps of liquid cultures inoculated 28Sep2014.
- Materials and Methods
- We performed 7 plasmid midipreps: 8 (bovine Beta casein B) A & B, 9 (FAM20C Kex +) A & B, 10 (FAM20C Kex +) A & B, and E (Electra cloning negative control.)
- We made stocks of 8B, 9B, and 10B --> 300uL 1:1 E. coli culture:50% glycerol. WE NEED TO TO THIS BEFORE EVERY MIDIPREP FROM NOW ON TO GENERATE STOCKS OF OUR PLASMIDS IN E. COLI
- We weren't able to generate stocks of 8A, 9A, 10A or the Electra negative, as those cultures had already been lysed.
- We used the Zyppy Plasmid Midiprep Kit (Protocol = Media:D4025i.pdf) with the following specifics:
- Began extraction process at 8:30 pm.
- Followed "Centrifugation Protocol" stream without the Step 1) "alternative" that we usually perform, as our E. coli cultures incubated longer than usual.
- Centrifugation of 50mL conical tubes took place in a Sorvall Legend T centrifuge.
- Centrifugation of 2mL kit spin columns and 2.0mL microfuge elution tubes took place in benchtop Beckman microfuge E.
- Removed 10uL eluted DNA for quantification. Removed 10uL for sequencing. Remaining 130uL DNA stored in Johan's pink box, BioCurious -20°C freezer. Tubes labeled with construct names, the date and "iGEM."
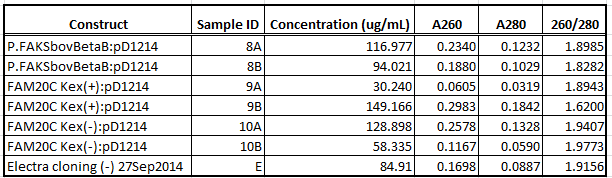
UV spec readings for plasmid DNA quantification & purity, 29Sep2014 midipreps
September 29, 2014
- Participants: Johan, Rachel
- Notes: Rachel
- Location: BioCurious
- Aims: Quantify and check purity ratio of bovine Beta casein B, FAM20C Kex (+), FAM20C Kex (-) in pD1214 (midiprepped as above, September 29, 2014) in preparation for submitting to Sequetech for sequencing.
- Materials & Methods: All measurements taken on BioCurious Beckman Coulter DU 640 spectrophotometer, DNA/Oligo setting, using 100ul cuvette.
Measuring plasmid DNA diluted 1:10:
Blank = 10ul Zyppy kit elution buffer + 90ul diH2O Sample = 10ul plasmid DNA + 90ul diH2O
- Results:
- Conclusions:
- DNA concentration decent except for 9A; 260/280 ratios tend a bit high ("pure" DNA = 1.8, "pure" RNA = 2.0).
- Constructs ready to send to Sequetech for sequencing.
- Sequencing notes in Submitting 10 casein and FAM20C in pD1214 plasmids for sequencing 30Sep2014.
</html>