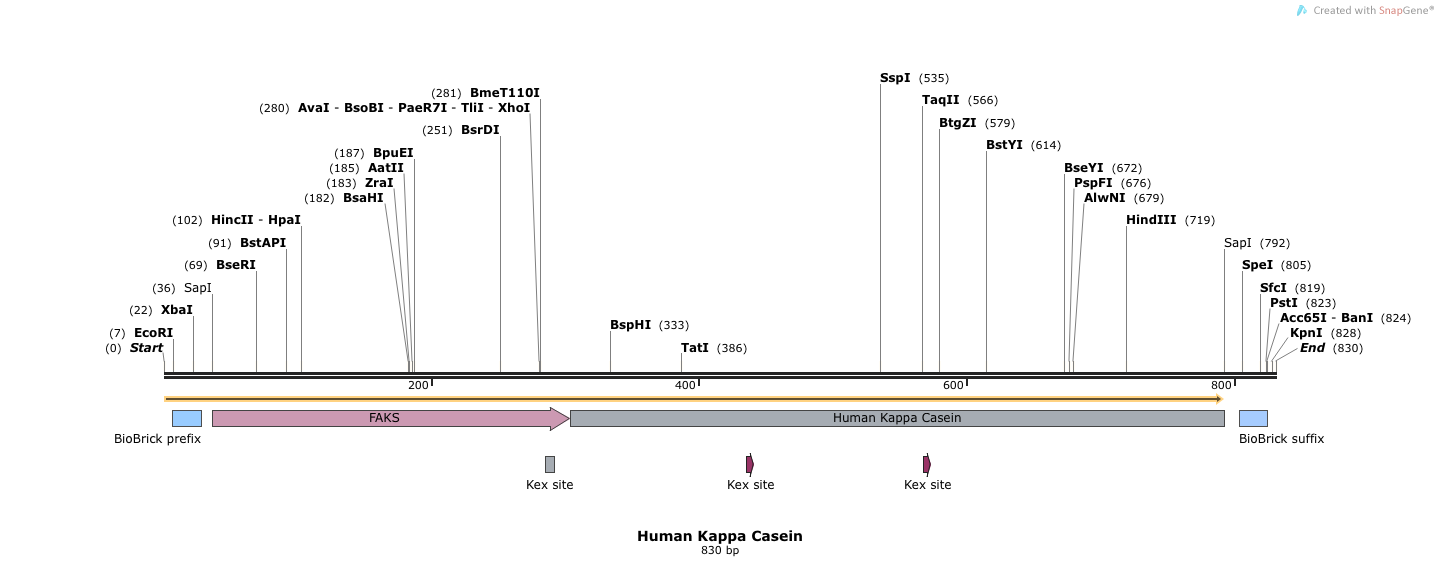
CLONE 082514 humKappaCasein(Kex+)
Revision as of 04:53, 2 September 2014 by Rachel Linzer (talk | contribs)
Experiment 082514_humKappaCasein(Kex+) Cloning
- Human Kappa Casein (BB.SapI.humKappa(Kex+).SapI.BB)
5'- TACACGGAATTCGCGGCCGCTTCTAGAGGCTCTTCTATGAGATTCCCATCTATTTTCACCGCTGTCTTGTTCGCTGCCTCCTCTGCATTGGCTGCCCCTGTTAAC ACTACCACTGAAGACGAGACTGCTCAAATTCCAGCTGAAGCAGTTATCGGTTACTCTGACCTTGAGGGTGATTTCGACGTCGCTGTTTTGCCTTTCTCTAACTCC ACTAACAACGGTTTGTTGTTCATTAACACCACTATCGCTTCCATTGCTGCTAAGGAAGAGGGTGTCTCTCTCGAGAAAAGAGAGGCCGAAGCTGAAGTCCAAAAC CAAAAGCAACCAGCTTGTCATGAAAACGACGAAAGACCATTCTACCAAAAGACTGCCCCATACGTTCCAATGTACTACGTTCCAAACTCTTACCCATACTACGGT ACTAACTTGTACCAAAGAAGACCTGCTATCGCCATTAACAACCCATACGTCCCAAGAACTTACTACGCTAATCCAGCTGTTGTTCGTCCACACGCTCAAATTCCA CAAAGACAATATTTGCCTAACTCTCACCCACCAACCGTTGTCAGAAGACCAAACTTGCATCCTTCTTTCATCGCTATCCCACCAAAAAAGATCCAAGACAAGATT ATCATCCCAACTATCAACACTATCGCCACCGTTGAACCAACCCCAGCTCCTGCCACCGAACCAACTGTCGATTCTGTTGTTACTCCAGAAGCTTTCTCCGAATCT ATCATTACTTCTACTCCAGAAACTACCACTGTCGCCGTCACCCCACCAACTGCTTAGGGTAGAAGAGCTACTAGTAGCGGCCGCTGCAGGTACCA - 3'
- This sequence has 5' and 3' SapI sequences, followed by full length FAKS, followed by human Kappa Casein (Kex+). Electra cloning cleaves at SapI sites and ligates simulatenously.
- We cloned into pD1214. This vector was ordered from DNA2.0 and is linearized upon arrival. Our insert is treated with Electra enzyme mix which cuts and ligates our "SapI.gene.SapI" and inserts it into the pD1214 vector.
- SapI treated DNA has an ATG overhang at the 5' end and a GGT overhang at the 3' end. Our complete vector will have the alphafactorfull signal sequence, which leads into another Methionine (M), followed by the rest of the human kappa casein protein.
All you need to know about the Electra system.
August 25, 2014
- Successfully cloned SapI.hKcasein.SapI into the electra daughter vector (pD1214).
Reaction: 1uL pD1214:FAKS (20ng) 1uL (20ng) of BB.SapI.humKappa(Kex+).SapI.BB 2uL Electra Buffer 1uL of Electra enzyme mix 15uL of Water ---- 20uL, Room Temperature, 20min.
- We stored the cloning reaction at -20 degrees C and waited to transform E. coli cells until August 26, 2014 (Nikola and Johan performed this) since we did not have competent cells available.
September 1, 2014: DNA quantification of humKappaCasein(Kex+) in pD1214
- Participants: Advait, Patrik, Johan, Minakishi, Rachel, Lafia, Mac
- Location: BioCurious
- Aims: Quantify and check purity ratio of humKappaCasein(Kex+)in pD1214 (midi prepped by XX using Zymo Research Zyppy midiprep kit, August XX, 2014) in preparation for submitting to Sequetech for sequencing.
- Materials & Methods: All measurements taken on BioCurious Beckman Coulter DU 640 spectrophotometer, DNA/Oligo setting, using 100ul cuvette.
Measuring plasmid DNA diluted 1:100 Blank = 1ul Zyppy kit elution buffer + 99ul diH2O Sample = 1ul plasmid DNA + 99ul diH2O
- Results: Spectrophotometer gave DNA concentration readings of 0. Repeating with a lower dilution factor
Measuring plasmid DNA diluted 1:10 Blank = 10ul Zyppy kit elution buffer + 90ul diH2O Sample = 10ul plasmid DNA + 90ul diH2O
- Results:
- Read 1: 42.158 ug/mL; 260/280 ratio = 1.9291
- Read 2: 42.433 ug/mL; 260/280 ratio = 1.9382
- Conclusions:
- DNA concentration looks good; 260/280 ratio a bit high ("pure" DNA = 1.8, "pure" RNA = 2.0).
- Construct ready to send to Sequetech for sequencing.