Difference between revisions of "Cloning strategy"
| (40 intermediate revisions by 4 users not shown) | |||
| Line 33: | Line 33: | ||
The shuttle vector will arrive linearized with overlaps for DNA 2.0's own Electra cloning system. | The shuttle vector will arrive linearized with overlaps for DNA 2.0's own Electra cloning system. | ||
[[File:PD1214-FAKS.png|470px]] [[File:Pd1214-faks.jpg|350px]] | |||
[[File:ElectrapDAUGHTERinsertion.png|828px]] | |||
== Reagents == | == Reagents == | ||
| Line 107: | Line 111: | ||
We don't have the sequences yet! | We don't have the sequences yet! | ||
=Experiments= | |||
*[[DNA Handling]] | |||
*[[CR_062214_humanKappaCasein_001]] | |||
*[[CLONE_082514_humKappaCasein(Kex+)]] | |||
=Progress summary, Milestones 1 & 2= | |||
{| class="wikitable" | |||
|- style="height: 40px;" | |||
! gBlock gene construct | |||
! Ordered, IDT | |||
! Received, IDT | |||
! Cloned into pD1214 | |||
shuttle vector | |||
! Plasmid construct | |||
transformed into ''E. coli'' | |||
! Plasmid prep | |||
! Construct | |||
sequence confirmed | |||
! BioBrick Part Number | |||
|- | |||
| P.FAKS.bovAlphaS1.S | |||
| Aug. 18, 2014 | |||
| Sep. 2, 2014 | |||
| Sep. 12, 2014 | |||
| Sep. 12, 2014 | |||
| Reprep. Oct. 3, 2014 | |||
Sep. 14, 2014 | |||
Ambig. seq. Resubmit | |||
and/or reprep new clone/s. | |||
| Oct. 13, 2014 | |||
| BBa_K1531000 | |||
|- | |||
| P.FAKS.bovAlphaS2(Kex+).S | |||
| Aug. 18, 2014 | |||
| Sep. 2, 2014 | |||
| Sep. 12, 2014 | |||
| Sep. 12, 2014 | |||
| Reprep. Oct. 3, 2014 | |||
Sep. 14, 2014 | |||
Wrong seq. Reprep | |||
new clone/s. | |||
| Oct. 13, 2014 | |||
| BBa_K1531001 | |||
|- | |||
| P.FAKS.bovAlphaS2(Kex-).S | |||
| Aug. 18, 2014 | |||
| Sep. 1, 2014 | |||
| Sep. 12, 2014 | |||
| Sep. 12, 2014 | |||
| Sep. 14, 2014 | |||
| Oct. 1, 2014 | |||
| BBa_K1531002 | |||
|- | |||
| P.FAKS.Beta(A2).S (bov) | |||
| Aug. 18, 2014 | |||
| Sep. 2, 2014 | |||
| Sep. 12, 2014 | |||
| Sep. 12, 2014 | |||
| Reprep. Oct. 3, 2014 | |||
Sep. 14, 2014 | |||
Ambig. seq. Resubmit | |||
[ | and/or reprep new clone/s. | ||
| Oct. 13, 2014 | |||
| BBa_K1531003 | |||
|- | |||
| P.FAKS.bovBeta(B).S | |||
| Aug. 18, 2014 | |||
| Sep. 16, 2014 | |||
| Sep. 27, 2014 | |||
| Sep. 27, 2014 | |||
| Sep. 29, 2014 | |||
| Oct. 1, 2014 | |||
| [http://parts.igem.org/Part:BBa_K1531004 BBa_K1531004] | |||
|- | |||
| P.FAKS.bovKappa.S | |||
| Aug. 18, 2014 | |||
| Sep. 2, 2014 | |||
| Sep. 12, 2014 | |||
| Sep. 12, 2014 | |||
| Sep. 14, 2014 | |||
| Oct. 1, 2014 | |||
| [http://parts.igem.org/Part:BBa_K1531005 BBa_K1531005] | |||
|-style="height: 40px;" | |||
| P.FAKS.humAlphaS1.S | |||
| Aug. 18, 2014 | |||
Redesigned & ordered | |||
Sep. 26, 2014 | |||
| Oct. 3, 2014 | |||
(Sep. 26 order) | |||
IDT synth. FAIL | |||
Sep. 4, 2014 | |||
| Oct. 4, 2014 | |||
| Oct. 4, 2014 | |||
| Oct. 9, 2014(?) | |||
| Oct. 13, 2014 | |||
| BBa_K1531006 | |||
|-style="height: 40px;" | |||
| P.FAKS.humBeta.S | |||
| Aug. 18, 2014 | |||
| Sep. 2, 2014 | |||
| Sep. 12, 2014 | |||
Sep. 15, 2014 | |||
= Rxn repeat | |||
| Sep. 12, 2014 = Rxn fail | |||
Sep. 14, 2014 = Rxn fail | |||
Sep. 15, 2014 = + colonies | |||
| Sep. 17, 2014 prep | |||
of Sep. 15 tfm | |||
| Oct. 1, 2014 | |||
| BBa_K1531007 | |||
|- | |||
| P.FAKS.humKappa(Kex+).S | |||
| Aug. 18, 2014 | |||
| Aug. 25, 2014 | |||
| Aug. 25, 2014 | |||
| Aug. 26, 2014 | |||
| Aug. 27, 2014 | |||
| Sep. 6, 2014 | |||
| BBa_K1531008 | |||
|- | |||
| P.FAKS.humKappa(Kex-).S | |||
| Aug. 18, 2014 | |||
| Sep. 1, 2014 | |||
| Sep. 12, 2014 | |||
| Sep. 12, 2014 | |||
| Reprep. Oct. 4, 2014 | |||
Sep. 14, 2014 | |||
Wrong seq. Reprep | |||
new clone/s. | |||
| | |||
| BBa_K1531009 | |||
|- | |||
| Sap.FAKS.hFam20C(Kex+).Sap | |||
| Aug. 18, 2014 | |||
| Sep. 16, 2014 | |||
| Sep. 27, 2014 | |||
| Sep. 27, 2014 | |||
| Sep. 29, 2014 | |||
| | |||
| BBa_K1531010 | |||
|- | |||
| Sap.Faks.hFam20C(Kex-).Sap | |||
| Aug. 18, 2014 | |||
| Sep. 24, 2014 | |||
| Sep. 27, 2014 | |||
| Sep. 27, 2014 | |||
| Sep. 29, 2014 | |||
| Oct. 15, 2014 | |||
| BBa_K1531011 | |||
|} | |||
Latest revision as of 07:06, 16 October 2014
Milestone
Our first milestone will be to express and secrete full-length kappa-casein in S. cerevisiae. We only get one discounted order of gBlocks from IDT so we will save that for later milestones and pay full price for the initial kappa-casein synthesis.
Once the first milestone has been accomplished, we will attempt express
Orders for first milestone
E. coli
- E. coli NEB-10: We have this strain available in our freezer.
S. cerevisiae
- The strain must be a URA3 knockout: We are requesting a donation from NEB
Shuttle vector
The shuttle vector is a vector that can be grown and selected in E. coli and S. cerevisiae, but which only correctly expresses the gene or genes of interest in S. cerevisiae.
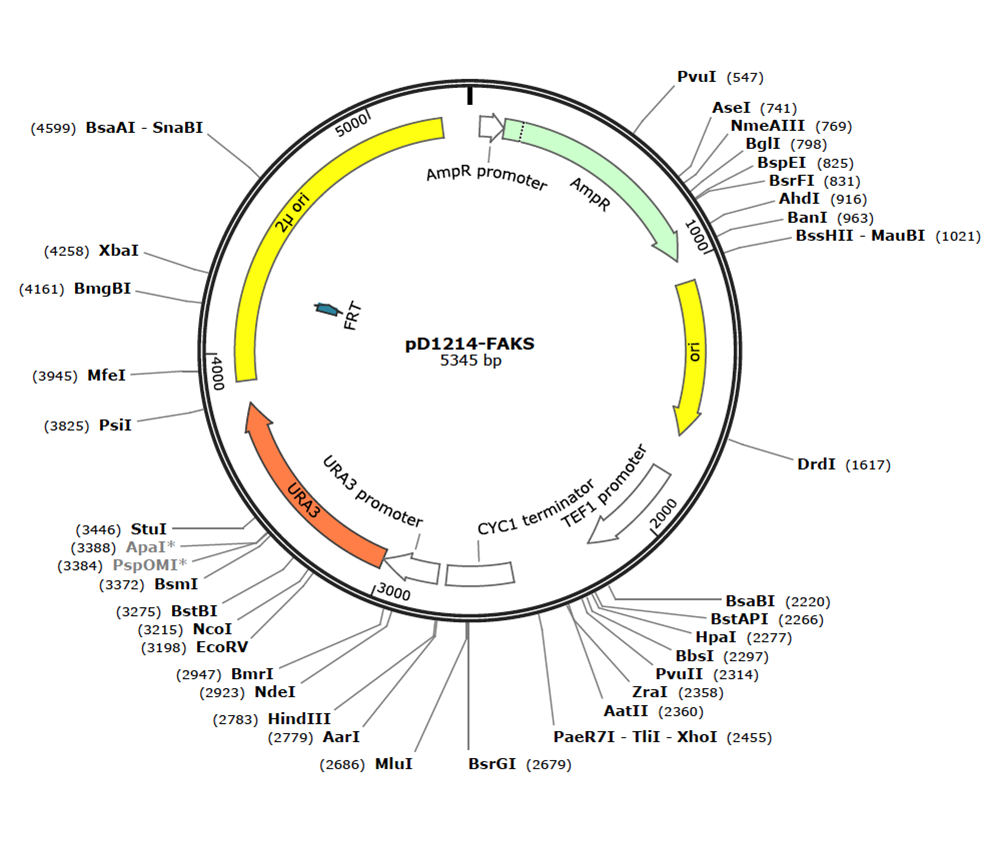
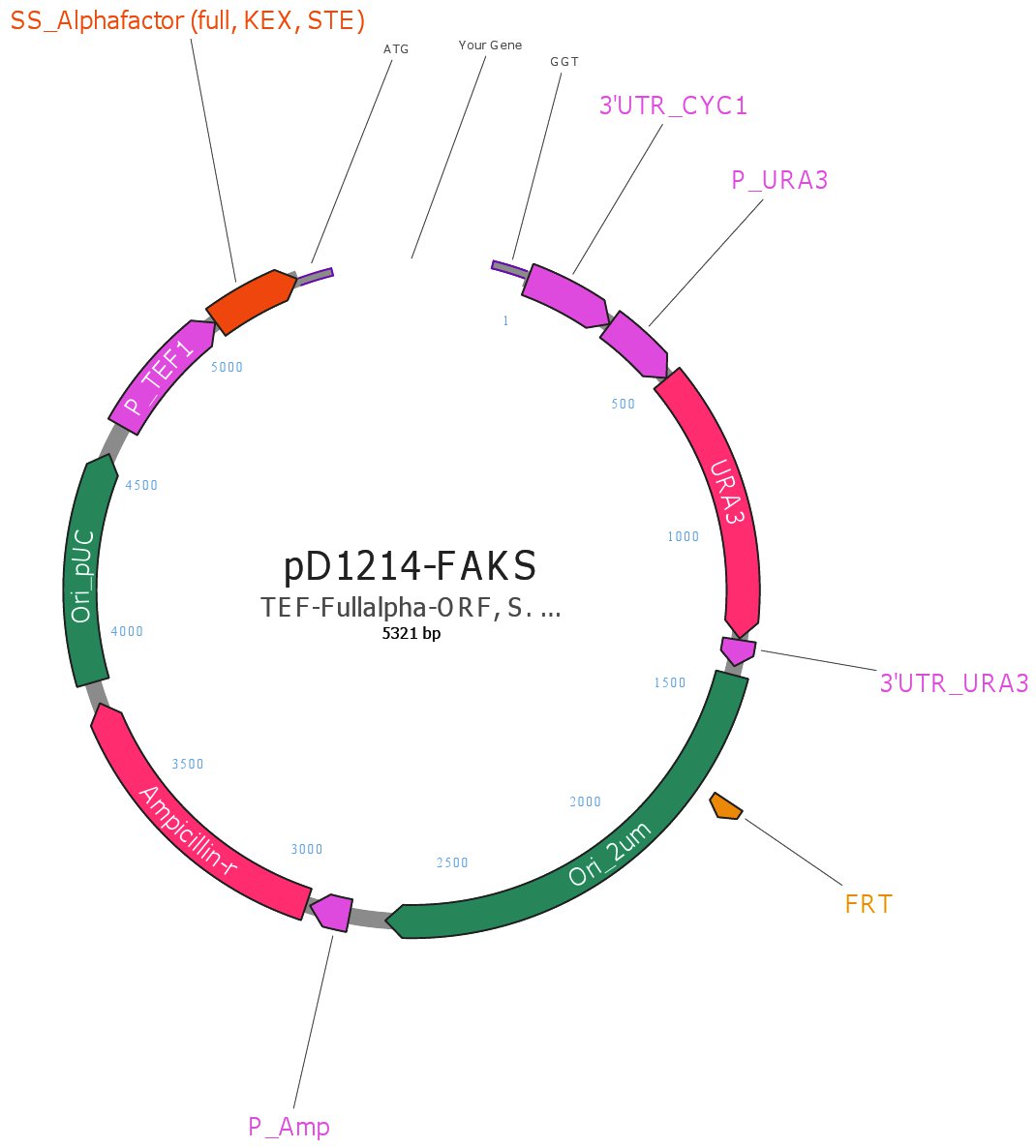
We're ordering the pD1214-FAKS shuttle vector from DNA 2.0 which contains the following features:
- AmpR: Ampicillin resistance gene (for selection in E. coli)
- P-Amp: Constitutive promoter for AmpR
- pUCori: High copy number E. coli origin of replication
- 2um: High copy number S. cerevisiae origin of replication from 2-μm plasmid.
- URA3: Yeast selectable marker. Requires the use of an S. cerevisiae strain missing the URA3 gene.
- P_ura: Constitutive S. cerevisiae promoter for URA3
- T_ura: S. cerevisiae terminator for URA3
- P_TEF1_syn: Constitutive S. cerevisiae promoter for our gene of interest (we will swap this out for an inducible promoter later)
- Secretion signal: Full alpha-factor yeast secretion signal. Fused to our gene of interest to secrete the protein.
- CYC1terminator: S. cerevisiae terminator for our gene of interest.
The shuttle vector will arrive linearized with overlaps for DNA 2.0's own Electra cloning system.
Reagents
Since we are using the DNA2.0 Elektra System to clone full-length Kappa-Casein, our reagents list is significantly shorter! Here it is:
Cloning Elektra Vector (Craig Donated) Elektra System (Craig Donated)
E. coli Cloning Carbenicillin (50mg/mL, 25mL) donated by Teknova LB Broth (1L) donated by Teknova LB Carb 50Plates (20) donated by Teknova NEB 10-beta Competent E. coli (High Efficiency)
Genes SapI.KappaCasein.SapI (human) (Craig Donated)
Saccharomyces Expression Saccharomyces cerevisae (Ura3ko)? UV-sensitive, other muts LiOAC (250kg) PEG (500mL, 50%) YPD Plates + uridine (Ura3- growth) YPD Plates (Ura3+ selection; animal free) YPD Broth + Uridine (80ug/mL; 25 tubes/rack) YPD Broth (animal Free Soytone, 1000mL) donated by Teknova
PPE Sterile Nitrile gloves (50/box) Sterile Nitrile gloves (50/box) Biohazard Bags
Consumables 20uL Pipette Tips 200uL Pipette tips 1000uL Tips 1.5mL Eppendorf PCR tubes Plasmid miniPrep
Orders for second milestone
This is a work in progress. So far we're planning a large order of gBlocks from IDT including at least some of the following sequences:
Bovine:
- Full-length bovine κ Casein
- B genetic variant AAQ87923.1
- Full-length bovine αs1 Casein
- C genetic variant ACG63494.1
- Full-length bovine αs2 Casein
- A variant P02663
- Full-length bovine β Casein
- A2 genetic variant for health benefit P02666.2
- B genetic variant for better coagulation AAI11173.1
Human:
- Full-length human κ Casein P07498.3
- Full-length human αs1 Casein
- P47710.1
- (There is no human αs2a Casein, just two truncated pseudogenes)
- Full-length human β Casein
- P05814.4
- human caseinomacropeptide (why?)
- FAM20C Kinase
- Q8IXL6
Whale:
We don't have the sequences yet!
Experiments
Progress summary, Milestones 1 & 2
| gBlock gene construct | Ordered, IDT | Received, IDT | Cloned into pD1214
shuttle vector |
Plasmid construct
transformed into E. coli |
Plasmid prep | Construct
sequence confirmed |
BioBrick Part Number |
|---|---|---|---|---|---|---|---|
| P.FAKS.bovAlphaS1.S | Aug. 18, 2014 | Sep. 2, 2014 | Sep. 12, 2014 | Sep. 12, 2014 | Reprep. Oct. 3, 2014
Sep. 14, 2014 Ambig. seq. Resubmit and/or reprep new clone/s. |
Oct. 13, 2014 | BBa_K1531000 |
| P.FAKS.bovAlphaS2(Kex+).S | Aug. 18, 2014 | Sep. 2, 2014 | Sep. 12, 2014 | Sep. 12, 2014 | Reprep. Oct. 3, 2014
Sep. 14, 2014 Wrong seq. Reprep new clone/s. |
Oct. 13, 2014 | BBa_K1531001 |
| P.FAKS.bovAlphaS2(Kex-).S | Aug. 18, 2014 | Sep. 1, 2014 | Sep. 12, 2014 | Sep. 12, 2014 | Sep. 14, 2014 | Oct. 1, 2014 | BBa_K1531002 |
| P.FAKS.Beta(A2).S (bov) | Aug. 18, 2014 | Sep. 2, 2014 | Sep. 12, 2014 | Sep. 12, 2014 | Reprep. Oct. 3, 2014
Sep. 14, 2014 Ambig. seq. Resubmit and/or reprep new clone/s. |
Oct. 13, 2014 | BBa_K1531003 |
| P.FAKS.bovBeta(B).S | Aug. 18, 2014 | Sep. 16, 2014 | Sep. 27, 2014 | Sep. 27, 2014 | Sep. 29, 2014 | Oct. 1, 2014 | BBa_K1531004 |
| P.FAKS.bovKappa.S | Aug. 18, 2014 | Sep. 2, 2014 | Sep. 12, 2014 | Sep. 12, 2014 | Sep. 14, 2014 | Oct. 1, 2014 | BBa_K1531005 |
| P.FAKS.humAlphaS1.S | Aug. 18, 2014
Redesigned & ordered Sep. 26, 2014 |
Oct. 3, 2014
(Sep. 26 order) IDT synth. FAIL Sep. 4, 2014 |
Oct. 4, 2014 | Oct. 4, 2014 | Oct. 9, 2014(?) | Oct. 13, 2014 | BBa_K1531006 |
| P.FAKS.humBeta.S | Aug. 18, 2014 | Sep. 2, 2014 | Sep. 12, 2014
Sep. 15, 2014 = Rxn repeat |
Sep. 12, 2014 = Rxn fail
Sep. 14, 2014 = Rxn fail Sep. 15, 2014 = + colonies |
Sep. 17, 2014 prep
of Sep. 15 tfm |
Oct. 1, 2014 | BBa_K1531007 |
| P.FAKS.humKappa(Kex+).S | Aug. 18, 2014 | Aug. 25, 2014 | Aug. 25, 2014 | Aug. 26, 2014 | Aug. 27, 2014 | Sep. 6, 2014 | BBa_K1531008 |
| P.FAKS.humKappa(Kex-).S | Aug. 18, 2014 | Sep. 1, 2014 | Sep. 12, 2014 | Sep. 12, 2014 | Reprep. Oct. 4, 2014
Sep. 14, 2014 Wrong seq. Reprep new clone/s. |
BBa_K1531009 | |
| Sap.FAKS.hFam20C(Kex+).Sap | Aug. 18, 2014 | Sep. 16, 2014 | Sep. 27, 2014 | Sep. 27, 2014 | Sep. 29, 2014 | BBa_K1531010 | |
| Sap.Faks.hFam20C(Kex-).Sap | Aug. 18, 2014 | Sep. 24, 2014 | Sep. 27, 2014 | Sep. 27, 2014 | Sep. 29, 2014 | Oct. 15, 2014 | BBa_K1531011 |