Difference between revisions of "Molecular biology"
| (93 intermediate revisions by 5 users not shown) | |||
| Line 2: | Line 2: | ||
= Intro = | = Intro = | ||
Kappa casein is the protein responsible for milk coagulation into cheese when it is cleaved by rennet | Kappa casein is the protein responsible for milk coagulation into cheese when it is cleaved by the [[chymosin]] enzyme in rennet. If we could express this protein in yeast, we could essentially make vegan cheese. | ||
[http://www.ncbi.nlm.nih.gov/pubmed/16078069 A 2005 paper] showed that the C-terminal part of the human kappa casein protein can be overexpressed in S. cerevisiae (baker’s yeast) and Pichia pastoris, and they were able to get it secreted in sufficient quantity that they could run the culture medium on a gel, and see a clear band for the protein. (“This macropeptide has various biological activities and is used as a functional food ingredient as well as a pharmaceutical compound.“ - valuable stuff, not just for vegan cheese!) | [http://www.ncbi.nlm.nih.gov/pubmed/16078069 A 2005 paper] showed that the C-terminal part of the human kappa casein protein can be overexpressed in S. cerevisiae (baker’s yeast) and Pichia pastoris, and they were able to get it secreted in sufficient quantity that they could run the culture medium on a gel, and see a clear band for the protein. (“This macropeptide has various biological activities and is used as a functional food ingredient as well as a pharmaceutical compound.“ - valuable stuff, not just for vegan cheese!) | ||
| Line 10: | Line 10: | ||
We can have some people working simultaneously on trying to express the other casein proteins (alpha-s1, alpha-s2, and beta). But since these are hydrophobic, we may not be able to express and secrete them without kappa-casein to hold them in suspension in a casein micelle. We can also attempt to modify the yeast lipid biosynthesis pathways to produce milk fats. | We can have some people working simultaneously on trying to express the other casein proteins (alpha-s1, alpha-s2, and beta). But since these are hydrophobic, we may not be able to express and secrete them without kappa-casein to hold them in suspension in a casein micelle. We can also attempt to modify the yeast lipid biosynthesis pathways to produce milk fats. | ||
= Essential reading = | |||
As recommended by Patrik: | |||
*[http://www.umb.no/statisk/nordost/laht_renneting_jurmala.pdf Rennet coagulation of milk] | *[http://www.umb.no/statisk/nordost/laht_renneting_jurmala.pdf Rennet coagulation of milk] | ||
| Line 16: | Line 18: | ||
*Production of human caseinomacropeptide in recombinant Saccharomyces cerevisiae and Pichia pastoris (on seafile) | *Production of human caseinomacropeptide in recombinant Saccharomyces cerevisiae and Pichia pastoris (on seafile) | ||
== | = Proteins = | ||
Patrik D says: Turns out casein proteins are not quite as insoluble as I had feared, especially if you keep the Ca concentration low enough. They've even been explored as chaperones to get other problematic proteins to fold better. There's also some papers on alpha- and beta-casein expression in E. coli and yeast, and on reconstitution of casein micelles from beta and kappa casein (beta casein is the major component in human milk). | |||
Exon-intron structure of bovine milk proteins: | |||
[[File:1-s2.0-S0022030209708653-gr1.jpg|link=http://www.sciencedirect.com/science/article/pii/S0022030209708653]] | |||
Organization of the casein genes in the human, mouse, rat, and cattle genome: | |||
[[File:RijnkelsGenomics03Fig1A.png]] | |||
== Milk composition == | |||
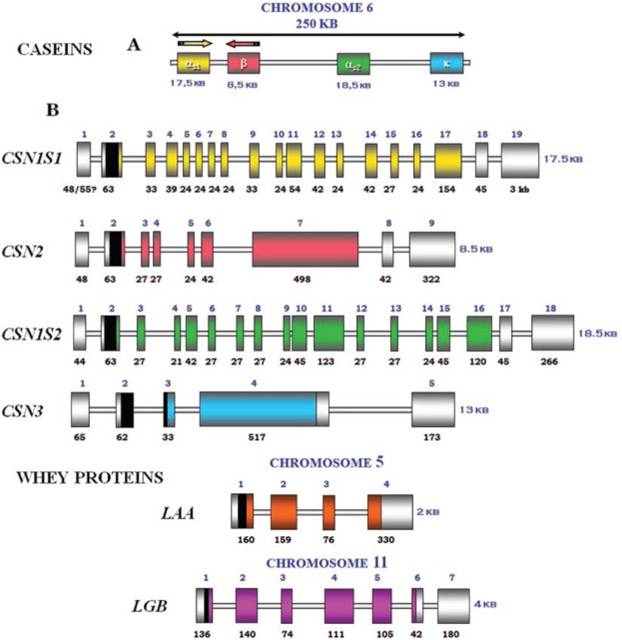
Mature bovine milk contains (as a percentage of total skim-milk proteins) 39-46% Alpha-s1-casein, 8-11% Alpha-s2-casein, 25-35% Beta-casein, 8-15% Kappa-casein, and 3-7% Gamma-casein (mostly fragments of Beta casein). Caseins make up about 81% of the total protein in bovine milk, but only 30% of the proteins of human milk. The dominant casein in human milk is Beta casein. | |||
== Alpha-s1 casein (CSN1S1) == | |||
[[File:Alpha-s1-casein.jpg|link=http://www.sigmaaldrich.com/life-science/metabolomics/enzyme-explorer/enzyme-reagents/casein.html]] | |||
Major component of cow's milk, along with beta casein. | |||
α-s1 Casein is the most prevalent form of casein in bovine milk. It has been reported to exhibit antioxidant and radical scavenging properties.1 It has also been reported to be involved in the transport of and casein from the endoplasmic reticulum to the Golgi apparatus. [Chanat, E., J. Cell Sci., 112, 3399-3412 (1999)] [http://www.sigmaaldrich.com/life-science/metabolomics/enzyme-explorer/enzyme-reagents/casein.html] | |||
=== Genetic variants & health effects === | |||
A recent review of milk protein nomenclature (Farrell et al., 2004) indicated 8 αs1-CN (A, B, C, D, E, F, G, H) [...] variants in the Bos genus. [http://www.ncbi.nlm.nih.gov/pubmed/19841193] | |||
CSN1S1 C positively affected milk coagulation [http://www.ncbi.nlm.nih.gov/pubmed/23746587] | |||
=== Sequences === | |||
*Bovine: [http://www.uniprot.org/uniprot/P02662 UniProt P02662] (A variant) | |||
*Human: [http://www.uniprot.org/uniprot/P47710 UniProt P47710] | |||
Bovine alpha-S1-casein is a 214 AA protein, including a 15 AA secretion signal. The molecular weight is 24,529 Da. | |||
Alpha-S1 is a key protein in milk protein allergy, and there is some data available on immunoglobulin E (IgE)-binding epitopes (i.e. antigenic peptides) in different genetic variants (Lisson et al., 2014), but there seems to be a lot of variability from patient to patient, and it is not clear (to me at least) what the health effects are of the different variants. | |||
Until we can figure out the health effects, I think we should focus on CSN1S1*C for its improved coagulation properties: | |||
>gi|195973860|gb|[http://www.ncbi.nlm.nih.gov/protein/ACG63494.1 ACG63494.1]| alpha S1 casein [Bos taurus] | |||
'''MKLLILTCLVAVALA'''RPKHPIKHQGLPQEVLNENLLRFFVAPFPEVFGKEKVNELSKDIGSESTEDQAME | |||
DIKQMEAESISSSEEIVPNSVEQKHIQKDDVPSERYLGYLEQLLRLKKYKVPQLEIVPNSAEERLHSMKE | |||
GIHAQQKEPMIGVNQELAYFYPELFRQFYQLDAYPSGAWYYVPLGTQYTDAPSFSDIPNPIGSENSGKTT | |||
MPLW | |||
Human alpha-S1-casein is present at much lower concentration than in bovine milk, and is only 185 AA, molecular weight 21,671 Da: | |||
>gi|1345671|sp|P47710.1|CASA1_HUMAN RecName: Full=Alpha-S1-casein; Contains: RecName: Full=Casoxin-D; Flags: Precursor | |||
'''MRLLILTCLVAVALA'''RPKLPLRYPERLQNPSESSEPIPLESREEYMNGMNRQRNILREKQTDEIKDTRNE | |||
STQNCVVAEPEKMESSISSSSEEMSLSKCAEQFCRLNEYNQLQLQAAHAQEQIRRMNENSHVQVPFQQLN | |||
QLAAYPYAVWYYPQIMQYVPFPPFSDISNPTAHENYEKNNVMLQW | |||
Alignment between the bovine reference sequence (A variant), bovine C variant, and human alpha-S1-casein (only 33% sequence identity): | |||
P02662 1 '''MKLLILTCLVAVALA'''RPKHPIKHQGLPQEVLNENLLRFFVAPFPEVFGKEKVNELS--------------KDIGSESTED 66 | |||
ACG63494 1 '''MKLLILTCLVAVALA'''RPKHPIKHQGLPQEVLNENLLRFFVAPFPEVFGKEKVNELS--------------KDIGSESTED 66 | |||
P47710 1 '''MRLLILTCLVAVALA'''RPKLPLRY---PERLQNPSESS---EPIPLESREEYMNGMNrqrnilrekqtdeiKDTRNESTQN 74 | |||
P02662 67 QAMEDIKQMEAESISSSEEIVPNSVEQKHIQKEDVPSERYLGYLEQLLRLKKYKVPQLEIVPNSAEERLHSMKEGIHAQQ 146 | |||
ACG63494 67 QAMEDIKQMEAESISSSEEIVPNSVEQKHIQKDDVPSERYLGYLEQLLRLKKYKVPQLEIVPNSAEERLHSMKEGIHAQQ 146 | |||
P47710 75 CVVAEPEKMESSISSSSEEMSLSKCA------------------EQFCRLNEYN--QLQLQAAHAQEQIRRMNENSHVQV 134 | |||
P02662 147 KEPMIGVNQELAYFYPELFRQFYQLDAYPSGAWYYVPLGTQYTDAPSFSDIPNPIGSENS'''''E'''''KTTMPL-W 214 | |||
ACG63494 147 KEPMIGVNQELAYFYPELFRQFYQLDAYPSGAWYYVPLGTQYTDAPSFSDIPNPIGSENS'''''G'''''KTTMPL-W 214 | |||
P47710 135 P-----------------FQQLNQLAAYPYAVWYY-PQIMQYVPFPPFSDISNPTAHENY'''''E'''''KNNVMLqW 185 | |||
== Alpha-s2 casein (CSN1S2) == | |||
[[File:Alpha-s2-casein.jpg|link=http://www.sigmaaldrich.com/life-science/metabolomics/enzyme-explorer/enzyme-reagents/casein.html]] | |||
=== Genetic variants & health effects === | |||
A recent review of milk protein nomenclature (Farrell et al., 2004) indicated [...] 4 αs2-CN (A, B, C, D) [...] variants in the Bos genus. [http://www.ncbi.nlm.nih.gov/pubmed/19841193] | |||
Proteolytic fragments of α-s2 Casein have been shown to exhibit antibacterial activity. Specifically the 39 amino acid casocidin-1 peptide fragment has been shown to inhibit E. coli and Staph. carnosis growth. [Zucht, H. D., FEBS Lett., 371, 185-188 (1995)] [http://www.sigmaaldrich.com/life-science/metabolomics/enzyme-explorer/enzyme-reagents/casein.html] | |||
=== Sequences === | |||
*Bovine: [http://www.uniprot.org/uniprot/P02663 UniProt P02663] (A variant) | |||
Bovine Alpha-s2 casein is a 222 AA protein, including a 15 AA secretion signal peptide. The molecular weight is 26,019 Da. | |||
>sp|P02663|CASA2_BOVIN Alpha-S2-casein OS=Bos taurus GN=CSN1S2 PE=1 SV=2 | |||
'''MKFFIFTCLLAVALA'''KNTMEHVSSSEESIISQETYKQEKNMAINPSKENLCSTFCKEVVR | |||
NANEEEYSIGSSSEESAEVATEEVKITVDDKHYQKALNEINQFYQKFPQYLQYLYQGPIV | |||
LNPWDQVKRNAVPITPTLNREQLSTSEENSKKTVDMESTEVFTKKTKLTEEEKNRLNFLK | |||
KISQRYQKFALPQYLKTVYQHQKAMKPWIQPKTKVIPYVRYL | |||
In human, the alpha-s2 casein gene is duplicated, but both copies ([http://www.ncbi.nlm.nih.gov/gene/286828 CSN1S2AP], [http://www.ncbi.nlm.nih.gov/gene/100337616 CSN1S2BP ]) are truncated shortly after the signal peptide. Therefore, these loci appear to be a pseudogenes. | |||
== Beta casein (CSN2) == | |||
[[File:Beta-casein.jpg|link=http://www.sigmaaldrich.com/life-science/metabolomics/enzyme-explorer/enzyme-reagents/casein.html]] | |||
Main component of cow's and human milk. | |||
β-Casein and its fragments have been implicated in a number of biological functions. The casoparan peptide has been reported to activate macrophage phagocytosis and peroxide release. Casohypotensin and casoparan may be involved in bradykinin regulation. Casohypotensin has also been shown to be a strong inhibitor of endo-oligopeptidase A, a thiol-activated protease capable of degrading bradykinin and neurotensin, and hydrolyzing enkephalin-containing peptides to produce enkephalins. β-Caseins are also a source of casomorphin peptides which exhibit opioid activity binding to opioid receptors. Casomorphins may be the hydrolysis product of dipeptidyl peptidase IV. [Miyamoto, Y., et al., Am. J. Physiol. Renal. Physiol., 252, 670-F677 (1987)] [http://www.sigmaaldrich.com/life-science/metabolomics/enzyme-explorer/enzyme-reagents/casein.html] | |||
=== Genetic variants & health effects === | |||
A recent review of milk protein nomenclature (Farrell et al., 2004) indicated [...] 12 β-CN (A1, A2, A3, B, C, D, E, F, G, H1, H2, I) [...] variants in the Bos genus. [http://www.ncbi.nlm.nih.gov/pubmed/19841193] | |||
"CSN2 B positively affected milk coagulation, whereas CSN2 A(2), in particular, had a negative effect" [http://www.ncbi.nlm.nih.gov/pubmed/23746587] | |||
= | "Populations, which consume milk containing high levels of β-casein A2 variant, have a lower incidence of cardiovascular disease and type 1 diabetes. Furthermore, consumption of milk with the A2 variant may be associated with less severe symptoms of autism and schizophrenia." [http://www.ncbi.nlm.nih.gov/pubmed/16403684] "Human milk, goat milk, sheep milk and other species’ milk contain beta-casein which is ‘A2 like’, because they have a proline at the equivalent position in their beta-casein chains." [http://www.betacasein.org/index.php?p=variants]. | ||
The | The health benefits of "A2 milk" (or rather the health risks associated with A1/B beta-casein) are thought to be due to beta-casomorphin 7 (BCM-7), one of a group of degradation peptides with a chain length of 4–11 amino acids (aa), all starting with tyrosine residue in position 60 (Kostyra et al. 2004). The A1 variant (as well as B, C, F, and G) has a histidine rather than Proline in position 67, resulting in more efficient proteolysis at that location by elastase, and a 4-fold higher BCM-7 level in hydrolysed A1 milk vs A2 milk ([https://www.ncbi.nlm.nih.gov/pubmed/17666771 Kaminski et al, 2007]). Human BCM's also show opioid activity ([https://www.ncbi.nlm.nih.gov/pubmed/3005882 Koch et al., 1985]), but may have different health effects due to some amino acid differences. | ||
*[http:// | === Sequences === | ||
*[http://www. | *Bovine: [http://www.uniprot.org/uniprot/P02666 UniProt P02666] (A2 variant) | ||
*Human: [http://www.uniprot.org/uniprot/P05814 UniProt P05814] | |||
Bovine beta-casein is a 224 AA protein, including a 15 AA secretion signal. The weight is 25,107 Da. We should try bovine A2 for the reputed health benefits, and B for improved milk coagulation. | |||
A2: | |||
>gi|115660|sp|[http://www.ncbi.nlm.nih.gov/protein/P02666.2 P02666.2]|CASB_BOVIN RecName: Full=Beta-casein; Contains: RecName: Full=Casoparan; Contains: RecName: Full=Antioxidant peptide; Contains: RecName: Full=Casohypotensin; Flags: Precursor | |||
'''MKVLILACLVALALA'''RELEELNVPGEIVESLSSSEESITRINKKIEKFQSEEQQQTEDELQDKIHPFAQT | |||
QSLVYPFPGPIPNSLPQNIPPLTQTPVVVPPFLQPEVMGVSKVKEAMAPKHKEMPFPKYPVEPFTESQSL | |||
TLTDVENLHLPLPLLQSWMHQPHQPLPPTVMFPPQSVLSLSQSKVLPVPQKAVPYPQRDMPIQAFLLYQE | |||
PVLGPVRGPFPIIV | |||
B: | |||
>gi|83406093|gb|[http://www.ncbi.nlm.nih.gov/protein/AAI11173.1 AAI11173.1]| CSN2 protein [Bos taurus] | |||
'''MKVLILACLVALALA'''RELEELNVPGEIVESLSSSEESITRINKKIEKFQSEEQQQTEDELQDKIHPFAQT | |||
QSLVYPFPGPIHNSLPQNIPPLTQTPVVVPPFLQPEVMGVSKVKEAMAPKHKEMPFPKYPVEPFTERQSL | |||
TLTDVENLHLPLPLLQSWMHQPHQPLPPTVMFPPQSVLSLSQSKVLPVPQKAVPYPQRDMPIQAFLLYQE | |||
PVLGPVRGPFPIIV | |||
Human beta-casein is a 226 AA protein, including a 15 AA secretion signal peptide. The molecular weight is 25,382 Da. | |||
>gi|115661|sp|P05814.4|CASB_HUMAN RecName: Full=Beta-casein; Flags: Precursor | |||
'''MKVLILACLVALALA'''RETIESLSSSEESITEYKQKVEKVKHEDQQQGEDEHQDKIYPSFQPQPLIYPFVE | |||
PIPYGFLPQNILPLAQPAVVLPVPQPEIMEVPKAKDTVYTKGRVMPVLKSPTIPFFDPQIPKLTDLENLH | |||
LPLPLLQPLMQQVPQPIPQTLALPPQPLWSVPQPKVLPIPQQVVPYPQRAVPVQALLLNQELLLNPTHQI | |||
YPVTQPLAPVHNPISV | |||
Alignment between bovine A2, bovine B, and human (55% identity over 72% of the sequence): | |||
P02666 1 '''MKVLILACLVALALA'''RELEELNVPGEIVESLSSSEESITRINKKIEKFQSEEQQQTEDELQDKIHPFAQTQSLVYPFPGP 80 | |||
AAI11173 1 '''MKVLILACLVALALA'''RELEELNVPGEIVESLSSSEESITRINKKIEKFQSEEQQQTEDELQDKIHPFAQTQSLVYPFPGP 80 | |||
P05814 1 '''MKVLILACLVALALA'''RET---------IESLSSSEESITEYKQKVEKVKHEDQQQGEDEHQDKIYPSFQPQPLIYPFVEP 71 | |||
P02666 81 I'''''P'''''NS-LPQNIPPLTQTPVVVPPFLQPEVMGVSKVKEAMAPKHKEMPFPKYPVEPFTE'''''S'''''QSLTLTDVENLHLPLPLLQSWM 159 | |||
AAI11173 81 I'''''H'''''NS-LPQNIPPLTQTPVVVPPFLQPEVMGVSKVKEAMAPKHKEMPFPKYPVEPFTE'''''R'''''QSLTLTDVENLHLPLPLLQSWM 159 | |||
P05814 72 I'''''P'''''YGfLPQNILPLAQ-PAVVLPVPQPEIMEVPKAKDTVYTKGRVMPVLKSPTIPFFD'''''P'''''QIPKLTDLENLHLPLPLLQPLM 150 | |||
P02666 160 HQPHQPLPPTVMFPPQSVLSLSQSKVLPVPQKAVPYPQRDMPIQAFLLYQEPVLGP------VRGPF-----PIIV 224 | |||
AAI11173 160 HQPHQPLPPTVMFPPQSVLSLSQSKVLPVPQKAVPYPQRDMPIQAFLLYQEPVLGP------VRGPF-----PIIV 224 | |||
P05814 151 QQVPQPIPQTLALPPQPLWSVPQPKVLPIPQQVVPYPQRAVPVQALLLNQELLLNPthqiypVTQPLapvhnPISV 226 | |||
= | == Kappa casein (CSN3) == | ||
[[File:Kappa-casein.jpg|link=http://www.sigmaaldrich.com/life-science/metabolomics/enzyme-explorer/enzyme-reagents/casein.html]] | |||
κ-Casein's orientation on the surface of the casein micelle functions as an interface between the hydrophobic interior caseins and the aqueous environment. During clotting of milk, hydrolysis by chymosin or rennin releases the water soluble fragment, para-k-casein and the hydrophobic caseinomacropeptide. [http://www.sigmaaldrich.com/life-science/metabolomics/enzyme-explorer/enzyme-reagents/casein.html] | |||
*[https://en.wikipedia.org/wiki/K-Casein Kappa-casein on wikipedia] | *[https://en.wikipedia.org/wiki/K-Casein Kappa-casein on wikipedia] | ||
*[http://www.uniprot.org/uniprot/P02668 Bovine kappa-casein precursor gene CSN3] | *[http://www.uniprot.org/uniprot/P02668 Bovine kappa-casein precursor gene CSN3] | ||
*[http://www.uniprot.org/uniprot/?query=family:%22kappa-casein+family%22 kappa-casein genes in other mammals] | *[http://www.uniprot.org/uniprot/?query=family:%22kappa-casein+family%22 kappa-casein genes in other mammals] | ||
=== Genetic variants & health effects === | |||
A recent review of milk protein nomenclature (Farrell et al., 2004) indicated [...] 11 κ-CN (A, B, C, E, F1, F2, G1, G2, H, I, J) [...] variants in the Bos genus. [http://www.ncbi.nlm.nih.gov/pubmed/19841193] | |||
* Casoxins A, B and C have opioid antagonist activity. Casoxin C binds to the complement C3a receptors. Casoplatelin inhibits platelet aggregation. [Lawrence K., Creamer, L. K., et al., Journal of Dairy Science, 81, 3004-3012 (1998)] [http://www.sigmaaldrich.com/life-science/metabolomics/enzyme-explorer/enzyme-reagents/casein.html] | |||
* The protein polymorphism at position 148-150 led to 2 different ACE inhibiting peptides. The peptide Val-Ser-Pro derived from CSN3*F1 showed the highest IC50 value (21.8) and is also known from maize (Miyoshi et al., 1991). The peptide Ala-Ser-Pro at the same position of the amino acid sequence but derived from the alleles CSN3*B and CSN3*C is not known for ACE inhibitory effects yet and showed an IC50 value of 242.3. | |||
:The genetic variation at amino acid position 96–99 produced 2 further ACE inhibitory peptides. In CSN3*C, the peptide Ala-His-His-Pro (IC50 = 847.6) was identified and at the same position the peptide Ala-Cys-His-Pro (IC50 = 360.7) was identified in CSN3*G2. These 2 peptides are not known for ACE inhibitory effects yet. | |||
:The dominant genetic variant of CSN3 in German cattle breeds is CSN3*A, whereas the B allele shows a high frequency in German Brown (0.479; Erhardt, 1989). Compared with CSN3*A, the B allele shows association with higher milk protein content and positive effects on coagulation properties and cheese yield (NgKwai-Hang and Grosclaude, 1992). In addition to these positive attributes, the present study shows another reason for preferring milk with the κ-casein allele B, because of the appearance of a new ACE inhibitory peptide at position 148–150 of the protein. [http://www.ncbi.nlm.nih.gov/pubmed/19389946] | |||
* CSN3 B positively affected milk coagulation [http://www.ncbi.nlm.nih.gov/pubmed/23746587] | |||
=== Caseinomacropeptide === | |||
Kim YJ, Oh YK, Kang W, Lee EY, Park S. Production of human caseinomacropeptide in recombinant Saccharomyces cerevisiae and Pichia pastoris. J Ind Microbiol Biotechnol. 2005 Sep;32(9):402-8. | |||
<blockquote>“Caseinomacropeptide is a polypeptide of 64 amino acid residues (106-169) derived from the C-terminal part of the mammalian milk k-casein. This macropeptide has various biological activities and is used as a functional food ingredient as well as a pharmaceutical compound. The gene encoding the human caseinomacropeptide (hCMP) was synthesized and expressed with an alpha-factor secretion signal in the two yeast strains, Saccharomyces cerevisiae and Pichia pastoris. The complete polypeptide of the recombinant hCMP was produced and secreted in a culture medium by both the strains, but the highest production was observed in S. cerevisiae with a galactose-inducible promoter. In a fed-batch bioreactor culture, 2.5 g/l of the recombinant hCMP was obtained from the S. cerevisiae at 97 h.” | |||
“Escherichia coli XL1-Blue (Stratagene, USA) was used for cloning and propagating genes. Host strains for recombinant hCMP were S. cerevisiae 2805 (his-, ura-) [...] For S. cerevisiae, pYIGP (containing a constitutive GAP promoter) or pYEGa (containing a galactose-inducible GAL promoter) was used.”</blockquote> | |||
=== Sequence === | === Sequence === | ||
*Bovine: [http://www.uniprot.org/uniprot/P02668 UniProt P02668] | |||
*Human: [http://www.uniprot.org/uniprot/P07498 UniProt P07498] | |||
Bovine kappa-casein is 190 AA of which the first 21 AA form a secretion signal: | Bovine kappa-casein is 190 AA of which the first 21 AA form a secretion signal. The molecular weight is 21,269 Da. The bovine reference sequence is Genbank [http://www.ncbi.nlm.nih.gov/nuccore/AY380228 AY380228], RefSeq [http://www.ncbi.nlm.nih.gov/protein/NP_776719 NP_776719], corresponding to the CSN*A allele. | ||
Because of improved coagulation time, and possible additional health benefits, we should focus on bovine genetic variant CSN3*B instead: | |||
>gi|36988716|gb|[http://www.ncbi.nlm.nih.gov/protein/AAQ87923.1 AAQ87923.1]| kappa casein [Bos taurus] | |||
'''MMKSFFLVVTILALTLPFLGA'''QEQNQEQPIRCEKDERFFSDKIAKYIPIQYVLSRYPSYGLNYYQQKPVA | |||
LINNQFLPYPYYAKPAAVRSPAQILQWQVLSNTVPAKSCQAQPTTMARHPHPHLSF''MAIPPKKNQDKTEI'' | |||
''PTINTIASGEPTSTPTIEAVESTVATLEASPEVIESPPEINTVQVTSTAV'' | |||
(secretion signal in '''bold'''; caseinomacropeptide in ''italic'') | |||
Human kappa-casein is 182 AA. The molecular weight is 20,305. The main human variant is: | |||
>gi|1705606|sp|P07498.3|CASK_HUMAN RecName: Full=Kappa-casein; Flags: Precursor | |||
'''MKSFLLVVNALALTLPFLAV'''EVQNQKQPACHENDERPFYQKTAPYVPMYYVPNSYPYYGTNLYQRRPAIA | |||
INNPYVPRTYYANPAVVRPHAQIPQRQYLPNSHPPTVVRRPNLHPSF''IAIPPKKIQDKIIIPTINTIATV'' | |||
''EPTPAPATEPTVDSVVTPEAFSESIITSTPETTTVAVTPPTA'' | |||
Alignment between bovine A, bovine B, and human (53% sequence identity): | |||
P02668 1 '''MMKSFFLVVTILALTLPFLGA'''QEQNQEQPIRCEKDERFFSDKIAKYIPIQYVLSRYPSYGLNYYQQKPVALINNQFLPYP 80 | |||
AAQ87923 1 '''MMKSFFLVVTILALTLPFLGA'''QEQNQEQPIRCEKDERFFSDKIAKYIPIQYVLSRYPSYGLNYYQQKPVALINNQFLPYP 80 | |||
P07498 1 -'''MKSFLLVVNALALTLPFLAV'''EVQNQKQPACHENDERPFYQKTAPYVPMYYVPNSYPYYGTNLYQRRPAIAINNPYVPRT 79 | |||
P02668 81 YYAKPAAVRSPAQILQWQVLSNTVPAKSCQAQPTTMARHPHPHLSFMAIPPKKNQDKTEIPTINTIASGEPTSTPT'''''T'''''EAV 160 | |||
AAQ87923 81 YYAKPAAVRSPAQILQWQVLSNTVPAKSCQAQPTTMARHPHPHLSFMAIPPKKNQDKTEIPTINTIASGEPTSTPT'''''I'''''EAV 160 | |||
P07498 80 YYANPAVVRPHAQIPQRQYLPN--------SHPPTVVRRPNLHPSFIAIPPKKIQDKIIIPTINTIATVEPTPAPA'''''T'''''EPT 151 | |||
P02668 161 ESTVATLE'''''D'''''SPEVIE-SPPEINTVQVTSTAV 190 | |||
AAQ87923 161 ESTVATLE'''''A'''''SPEVIE-SPPEINTVQVTSTAV 190 | |||
P07498 152 VDSVVTPE'''''A'''''FSESIItSTPETTTVAVTPPTA 182 | |||
=== Post-translational modification === | === Post-translational modification === | ||
| Line 78: | Line 242: | ||
* Glycosylation of six residues, all of them Threonine ([https://en.wikipedia.org/wiki/O-linked_glycosylation#O-N-acetylgalactosamine_.28O-GalNAc.29 (O-GalNAc)] | * Glycosylation of six residues, all of them Threonine ([https://en.wikipedia.org/wiki/O-linked_glycosylation#O-N-acetylgalactosamine_.28O-GalNAc.29 (O-GalNAc)] | ||
* Phosphorylation of three residues, all of them Serine | * Phosphorylation of three residues, all of them Serine | ||
* One disulphide bond between two cysteine residues | * One disulphide bond between two cysteine residues, potentially important for multimerization [http://www.ncbi.nlm.nih.gov/pubmed/1628650] | ||
* One "Pyrrolidone carboxylic acid" modification to a glutamine (?) | * One "Pyrrolidone carboxylic acid" modification to a glutamine (?) | ||
| Line 95: | Line 259: | ||
</blockquote> | </blockquote> | ||
===Role of phosphorylation and glycosylation in micelle formation or coagulation=== | |||
See our separate page on [[Phosphorylation and glycosylation of caseins]] for a more in-depth look at the literature. | |||
2-Dimensional electrophoresis (2-DE) can be used to distinguish κ-CN isoforms. In a study of milk from Holstein-Friesian and Jersey cows, about 1/3 of kappa-casein was glycosylated, and almost all phosphorylated. The dominant isoform was 1P (40-50%), with 2P, 1P+1G, 1P+2G and 1P+3G each around 10-15%. [http://www.sciencedirect.com/science/article/pii/S0022030212002652] | |||
:'''studies on the relationship between κ-CN glycosylation and MCP [Milk Coagulation Properties] provide inconsistent results.''' Cases et al. (2003) estimated a favorable effect of κ-CN deglycosylation on acid coagulation of milk, with deglycosylated milk exhibiting gel formation at greater pH and increased gel stiffness compared with untreated milk. According to Robitaille et al. (1993) and Mariani et al. (1995), κ-CN glycosylation favorably affects both the rate of firming and the firmness of the curd, but its effects on coagulation time are trivial. Jensen et al. (2012b) described favorable effects of the glycosylated κ-CN fraction on rennet coagulation but, in a later study ( Jensen et al., 2012a), detected no significant difference in the κ-CN posttranslational modification pattern between samples exhibiting good or poor coagulation properties. [...] | |||
:Results from this study indicate that variation in RCT [Rennet Coagulation Time] attributable to κ-CNtot must be entirely ascribed to the glycosylated component of the protein. [...] | |||
:The degree of κ-CN glycosylation is a key determinant of protein hydrophobicity (O’Connell and Fox, 2000) [...] From a theoretical perspective, an increased degree of glycosylation is thus expected to stabilize the micelle structure (Dziuba and Minkiewicz, 1996) and to increase the time needed by chymosin to activate milk coagulation. However, there is no conclusive evidence that κ-CN is a key element for stability of the native caseinate emulsions [...] [http://www.sciencedirect.com/science/article/pii/S0022030214000940] | |||
== Casein Kinase == | |||
There are some confusing factors when talking about casein kinase. There are enzymes in most eukaryotes called [https://en.wikipedia.org/wiki/Casein_kinase Casein Kinase 1 and 2]. These apparently don't have much to do with casein: | |||
<blockquote> | |||
The name casein kinase 2 arose from the fact that casein is a convenient protein substrate that is generally used to assay its activity ... many researchers that work with this enzyme agreed to abandon the reference to casein because it leads to confusion about a possible physiological role for this substrate. The name protein kinase CK2 was recommended. -- [http://www.ncbi.nlm.nih.gov/pubmed/7896000 allende 1995] | |||
</blockquote> | |||
In fact, until 2012 the kinases responsible for casein phosphorylation were unknown. The actual kinase is a secreted kinase called Fam20C ([http://www.ncbi.nlm.nih.gov/pubmed/22582013 Tagliabracci et al. 2012]). | |||
Fam20C specifically recognizes S-x-E/pS (where x is any amino acid and E/pS can be Glu or phosphoserine). | |||
=== Gene === | |||
The gene is also known as DMP4. The human version is 584 AA long (including the signal peptide). The molecular weight is 66,234 Da. | |||
* [http://www.uniprot.org/uniprot/Q8IXL6 The human Fam20C sequence] | |||
* [http://www.uniprot.org/uniprot/Q5MJS3 The mouse Fam20C sequence] | |||
* [http://www.uniprot.org/uniprot/?query=DMP4&sort=score Gene searc on uniprot] | |||
* [https://en.wikipedia.org/wiki/FAM20C Wikipedia article] | |||
=== Required minerals/ions === | |||
Fam20C phosphorylated ... [''one of the sites phosphorylated in b-casein''] ... in the presence of MnCl2 and CoCl2 more efficiently than in the presence of MgCl2 and CaCl2. | |||
== Relevant articles == | |||
*[http://www.ncbi.nlm.nih.gov/pubmed/22582013 Tagliabracci et al. 2012] | |||
*[http://www.ncbi.nlm.nih.gov/pubmed/23276407 Tagliabracci et al. 2013] (review) | |||
=== Older notes and more info === | |||
Two species of casein kinase from lactating bovine mammary gland have been identified; a Ca2+- and CM-independent casein kinase and a Ca2+- and CM-dependent casein kinase. The Ca2+- and CM-independent casein kinase phosphorylates previously dephosphorylated αs1-, β- or κ-casein while the Ca2+- and CM-dependent casein kinase prefers previously dephosphorylated β- or κ-casein as substrates. Two activities are indicated by their substrate specificity, sensitivity to Ca2+ and CM, pH maxima, and differential solubilization by anionic detergents. The presence of a regulated casein kinase in the lactating mammary gland suggests that casein phosphorylation may be a regulator of micelle formation or secretion.[http://www.ncbi.nlm.nih.gov/pubmed/2917658] | |||
<blockquote> | |||
Fam20C appears to be the Golgi casein kinase that phosphorylates secretory pathway proteins within S-x-E motifs. Fam20C phosphorylates the caseins and several secreted proteins implicated in biomineralization | |||
[Casein kinase-1 and casein kinase-2] are physiologically unrelated to casein because they are mainly cytosolic and nuclear proteins that would be unlikely to encounter casein in the secretory pathway and have therefore been renamed protein kinase CK1 and protein kinase CK2. A physiological casein kinase activity has been characterized from highly enriched Golgi fractions of lactating mammary gland, liver, brain, and kidney and named Golgi-enriched fraction casein kinase (GEF-CK). The GEF-CK specifically recognizes the consensus S-x-E/pS (where x is any amino acid and E/pS can be Glu or phosphoserine). [...] A peptide substrate reproducing one of the sites phosphorylated in β-casein, KKIEKFQSE-EQQQ (β28-40), is selectively phosphorylated by GEF-CK. [...] Protein immunoblotting of immunoprecipitates from conditioned medium of U2OS cells overexpressing V5-tagged αs1-casein with FLAG-tagged Fam20C revealed a mobility shift in αs1-casein that was absent when αs1-casein was coexpressed with the inactive Fam20C D478A mutant [...] Thus, Fam20C appears to phosphorylate casein in vitro and in mammalian cells. -- [http://www.ncbi.nlm.nih.gov/pubmed/22582013/ source] | |||
</blockquote> | |||
Woah. That changes things. The [http://www.uniprot.org/uniprot/P02662 uniprot record for alpha-s1] shows that all phosphorylated residues except one follow the S-x-E/pS pattern. If this is true for the other casein proteins, then this Fam20C is likely what we want, and not casein kinase II. ([[User:Juul|Juul]] ([[User talk:Juul|talk]])) | |||
Beta-casein expressed in E. coli has been successfully phosphorylated by co-expression of casein kinase II: [http://www.ncbi.nlm.nih.gov/pubmed/9226716 Expression and characterization of phosphorylated recombinant human beta-casein in Escherichia coli]. We do not know if CK2 will also work correctly for alpha-casein (for which phosphorylation is important in micelle formation) nor for kappa-casein (where phosphorylation is probably less important), but there is probably a good chance that it does. More research needed. | |||
S. cerevisiae [http://www.ncbi.nlm.nih.gov/pubmed/2196445 does have Casein Kinase II], but it is somewhat different from bovine CK2. Here's a comparison: | |||
*Casein kinase II subunit alpha: [http://www.yeastgenome.org/cgi-bin/locus.fpl?dbid=S000001297 yeast] vs. [http://www.uniprot.org/uniprot/P68399 cow]: [http://www.uniprot.org/align/2014042242E9E3X1WP ~49% identity] | |||
*Casein kinase II subunit alpha': [http://www.yeastgenome.org/cgi-bin/locus.fpl?dbid=S000005587 yeast] vs. [http://www.uniprot.org/uniprot/P20427 cow]: [http://www.uniprot.org/align/2014042252FVK46SWB ~54% identity] | |||
*Casein kinase II subunit beta: [http://www.yeastgenome.org/cgi-bin/locus.fpl?dbid=S000002987 yeast] vs. [http://www.uniprot.org/uniprot/P67868 cow]: [http://www.uniprot.org/align/2014042242E9F4S3JF ~32% identity] | |||
*Casein kinase II subunit beta': [http://www.yeastgenome.org/cgi-bin/locus.fpl?dbid=S000005565 yeast] vs. [http://www.uniprot.org/uniprot/P67868 cow]: [http://www.uniprot.org/align/2014042252FVL2L02T ~38% identity] | |||
:Cow does not have a beta' so this last comparison is between yeast beta' and cow beta. | |||
Is there any way we can gauge if the yeast CK2 is likely to work? If we co-express bovine CK2, how might that affect the yeast and the casein? | |||
=== Sequence === | |||
*Bovine: [http://www.uniprot.org/uniprot/F1MXQ3 UniProt F1MXQ3] (unreviewed) | |||
*Human: [http://www.uniprot.org/uniprot/Q8IXL6 UniProt Q8IXL6] | |||
The Human FAM20C protein is 584 AA, including a 22 AA signal peptide: | |||
>gi|327478506|sp|Q8IXL6.2|DMP4_HUMAN RecName: Full=Extracellular serine/threonine protein kinase FAM20C; AltName: Full=Dentin matrix protein 4; Short=DMP-4; AltName: Full=Golgi-enriched fraction casein kinase; Short=GEF-CK; AltName: Full=Protein FAM20C; Flags: Precursor | |||
'''MKMMLVRRFRVLILMVFLVACA'''LHIALDLLPRLERRGARPSGEPGCSCAQPAAEVAAPGWAQVRGRPGEP | |||
PAASSAAGDAGWPNKHTLRILQDFSSDPSSNLSSHSLEKLPPAAEPAERALRGRDPGALRPHDPAHRPLL | |||
RDPGPRRSESPPGPGGDASLLARLFEHPLYRVAVPPLTEEDVLFNVNSDTRLSPKAAENPDWPHAGAEGA | |||
EFLSPGEAAVDSYPNWLKFHIGINRYELYSRHNPAIEALLHDLSSQRITSVAMKSGGTQLKLIMTFQNYG | |||
QALFKPMKQTREQETPPDFFYFSDYERHNAEIAAFHLDRILDFRRVPPVAGRMVNMTKEIRDVTRDKKLW | |||
RTFFISPANNICFYGECSYYCSTEHALCGKPDQIEGSLAAFLPDLSLAKRKTWRNPWRRSYHKRKKAEWE | |||
VDPDYCEEVKQTPPYDSSHRILDVMDMTIFDFLMGNMDRHHYETFEKFGNETFIIHLDNGRGFGKYSHDE | |||
LSILVPLQQCCRIRKSTYLRLQLLAKEEYKLSLLMAESLRGDQVAPVLYQPHLEALDRRLRVVLKAVRDC | |||
VERNGLHSVVDDDLDTEHRAASAR | |||
== Genetic variants == | |||
Excellent review: Milk protein polymorphisms in cattle: Effect on animal breeding and human nutrition. [http://www.ncbi.nlm.nih.gov/pubmed/19841193] | |||
"Genetic variants can result from SNP, as well as nucleotide (nt) deletions or insertions. A recent review of milk protein nomenclature (Farrell et al., 2004) indicated 8 αs1-CN (A, B, C, D, E, F, G, H), 4 αs2-CN (A, B, C, D), 12 β-CN (A1, A2, A3, B, C, D, E, F, G, H1, H2, I), 11 κ-CN (A, B, C, E, F1, F2, G1, G2, H, I, J), 11 β-LG (A, B, C, D, E, F, G, H, I, J, W), and 3 α-LA (A, B, C) variants in the Bos genus." | |||
"Substantial variation in milk coagulation properties has been observed among dairy cows. [...] the cows were genotyped for major genetic variants in the αS1- (CSN1S1), β- (CSN2), and κ-casein (CSN3) genes, revealing distinct differences in variant frequencies among breeds. [...] CSN1S1 C, CSN2 B, and CSN3 B positively affected milk coagulation, whereas CSN2 A(2), in particular, had a negative effect" [http://www.ncbi.nlm.nih.gov/pubmed/23746587] | |||
= Host organism = | |||
Which organism should we use? We've mostly assumed we were going with S. cerevisiae but Pichia pastoris might be preferable. [http://www.ncbi.nlm.nih.gov/pubmed/22454110 Here is a review comparing the two]. | |||
== P. pastoris == | |||
*[https://en.wikipedia.org/wiki/Pichia_pastoris wikipedia article] | |||
=== Advantages over S. cerevisiae === | |||
*Likely to have fewer problems with efficient protein secretion | |||
*It can grow to very high cell densities | |||
*It can grow on methanol which is cheap | |||
**This is kinda funny, since we can make methanol from methane (cow-farts) which means that our organism can eat the major greenhouse gas produced by cows. | |||
<blockquote> | |||
"Pichia can grow to very high cell densities, and under ideal conditions can multiply to the point where the cell suspension is practically a paste. As the protein yield from expression in a microbe is roughly equal to the product of the protein produced per cell and the number of cells, this makes Pichia of great use when trying to produce large quantities of protein without expensive equipment" | |||
-- wikipedia quoting Cregg et al. "Expression in the yeast Pichia pastoris" 2009 | |||
</blockquote> | |||
=== Potential problems === | |||
*It is unclear if it is safe for human consumption (meaning more purification needed) | |||
*Fewer resources available | |||
== S. cerevisiae == | |||
=== Advantages === | |||
*Familiar as a human consumable | |||
*FDA GRAS status | |||
**We can probably get away with less or no purification and still have a food-safe product | |||
=== Potention problems === | |||
<blockquote> | |||
"Although S. cerevisiae has a well-developed eukaryotic secretory pathway, it is not the most efficient yeast for high-level export of proteins to the extracellular medium." | |||
"...often transport to the extracellular medium is inefficient, especially for complex mammalian proteins of high molecular weight." | |||
-- Overview of Protein Expression in Saccharomyces cerevisiae by Strausberg and Strausberg | |||
</blockquote> | |||
=== Strain selection === | |||
The Johns Hopkins' team used [http://www.ncbi.nlm.nih.gov/pubmed/2659436 YPH500] | |||
*[http://wiki.yeastgenome.org/index.php/Commonly_used_strains List of commonly used lab strains] | |||
*[http://www.ncbi.nlm.nih.gov/pubmed/10862876 A comparison of four strains] (article access?) | |||
Carolina yeast sensitive yeast strain | |||
: [http://www.carolina.com/yeast-genetics/saccharomyces-cerevisiae-uv-sensitive-strain-g948-1cu-alpha-rad1-rad18-phr1-ura3-mutant-in-excision-repair/173634.pr?catId=&mCat=&sCat=&ssCat=&question=UV+sensitive+yeast Saccharomyces cerevisiae, UV-Sensitive Strain, G948-1C/U, alpha, rad1 rad18 phr1 ura3 mutant in excision repair] | |||
ATCC protease negative strain: | |||
=== Vectors === | |||
The Jons Hopkins iGEM team modified [http://2011.igem.org/Team:Johns_Hopkins/Project/Vector pRS400-series vectors], removing the MCS and replacing them with biobrick prefix and suffix. | |||
Someone (not Patrik) said that galactose induction is the most common form of controlling heterologous expression. Common vectors are the pYES2 and pESC series vectors. | |||
=== Secretion === | |||
Secretion of proteins will require a secretion signal (triggering processing through the ER, golgi and then exocytosis). | |||
=== | === BioBricks Parts Registry === | ||
*[http://2011.igem.org/Team:Johns_Hopkins VitaYeast project, by Johns Hopkins’ 2011 iGEM team] | |||
** Characterized promoters and terminators | |||
*[http://parts.igem.org/Yeast Yeast parts in the BioBricks parts registry] | |||
==== Plasmid backbones ==== | |||
The BioBricks parts registry contains [http://parts.igem.org/Yeast#Plasmid_backbones 17 plasmid backbones], including 9 listed as "[http://parts.igem.org/Help:Availability_and_usefulness Available and Working]". | |||
Most of these plasmid backbones are based on pRS315 or pRS305 comply with the AarI part assembly standard developed by the Lim lab. AarI is a rare 7-cutter Type IIS restriction enzyme that cuts 4bp offset from its binding site, and can be used for scarless ligation of up to 3 parts (+ backbone) simultaneously. For more information, see: | |||
* '''[http://2008.igem.org/Everything_you_ever_wanted_to_know_about_AarI Everything you ever wanted to know about AarI]''' | |||
* [http://2008.igem.org/wiki/images/5/5d/Design_AarI_primers.pdf Design AarI primers] | |||
* [http://2008.igem.org/wiki/images/0/0f/How_to_AarI_clone.pdf How to AarI clone] | |||
==== Promoters ==== | |||
The BioBricks parts registry contains [http://parts.igem.org/Yeast#Promoters 44 yeast promoters], including: | |||
* [http://parts.igem.org/Yeast#Constitutive 14 Constitutive] | |||
* [http://parts.igem.org/Yeast#Positively_regulated 19 Positively regulated] | |||
* [http://parts.igem.org/Yeast#Repressible 6 Repressible] | |||
* [http://parts.igem.org/Yeast#Multiply_regulated 5 Multiply regulated]. | |||
=== Other notes === | |||
pESC contains a bidirectional promoter which may allow expression of both parts of the protein without cleavage being required. How? | |||
= Published casein expression papers = | |||
Is there a common thread in published casein expression work? Do they use similar secretion signals, promoters, etc? | |||
== Kim et al., 2005: Production of human caseinomacropeptide in recombinant Saccharomyces cerevisiae and Pichia pastoris == | |||
The 64 AA (106-169) caseinomacropeptide of human kappa-casein was expressed and secreted at 120mg/L in S. cerevisiae from an inducible promoter. Expression from consitutive promoter, and expression in Pichia pastoris resulted in lower amounts of secreted protein. | |||
Yeast: S. cerevisiae 2805 (MATα pep4::HIS3 pro1-d can1 GAL2 his3 d ura3–52) | |||
Plasmid: pYEGα (Inducible, GAL 10 promoter, URA 3 marker, ampicillin resistance) | |||
pYEGα is based on YEp352, a multicopy number yeast-E.coli shuttle vector containing pBR322 sequence, yeast URA3 and yeast 2u replication origin. (Sohn et al., 1991) | |||
"The gene for the α-factor secretion signal sequence was placed before the hCMP structural gene so that the signal peptide was fused to the 5' end of the hCMP and that the recombinant hCMP was secreted in the culture medium. In addition, the Kozak sequence was inserted into the position preceding the ATG initiation codon to facilitate an efficient translation in yeast." (see also Figure 2 in Sohn et al., 1991) | |||
= Estimating amount of expressed protein = | |||
In the 2005 Kim et al. paper where they express caseinomacropeptide they describe using densitometry to estimate amount of expressed protein. They run the stained protein on an SDS-PAGE and use a gel imager to estimate amount of protein. There is an interesting article on how to use a normal scanner for densitometry [http://www.ncbi.nlm.nih.gov/pubmed/9059824 here]. We'd need at least of couple of calibration points which could be known amounts of a different protein. We'd have to know if the stain shows in equal amounts for the different proteins (per mole or per gram). | |||
= Casein micelles and cheese curd coagulation = | |||
Casein micelles are an essential component of milk, and cheese making. Curd formation is due to coagulation of casein micelles into a 3D network (a gel) that then traps any fat globules as well. So, no micelles - no cheese! | |||
*[https://www.ncbi.nlm.nih.gov/pubmed/18576490 Kinetics of milk coagulation: II. Kinetics of the secondary phase: micelle flocculation.] (Carlson et al, 1987) | *[https://www.ncbi.nlm.nih.gov/pubmed/18576490 Kinetics of milk coagulation: II. Kinetics of the secondary phase: micelle flocculation.] (Carlson et al, 1987) | ||
| Line 105: | Line 471: | ||
If the model proposed by Home is correct, then micelle formation will require alpha, beta, kappa-casein and colloidal calcium. The model also implies that the phosphoserine residues of both alpha and kappa-casein play an important role in micelle formation. | If the model proposed by Home is correct, then micelle formation will require alpha, beta, kappa-casein and colloidal calcium. The model also implies that the phosphoserine residues of both alpha and kappa-casein play an important role in micelle formation. | ||
Various groups have resynthesized casein micelles from purified beta and kappa casein | |||
== Synthetic casein micelles == | |||
Some researchers have created synthetic casein micelles. We should study their efforts: | Some researchers have created synthetic casein micelles. We should study their efforts: | ||
| Line 111: | Line 479: | ||
*[http://www.ncbi.nlm.nih.gov/pubmed/469064 Sub-structure of synthetic casein micelles] (Knoop et al, 1979) | *[http://www.ncbi.nlm.nih.gov/pubmed/469064 Sub-structure of synthetic casein micelles] (Knoop et al, 1979) | ||
*[http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=5160164 A phosphate-induced sub-micelle-micelle equilibrium in reconstituted casein micelle systems] (Slattery, 1979) | *[http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=5160164 A phosphate-induced sub-micelle-micelle equilibrium in reconstituted casein micelle systems] (Slattery, 1979) | ||
*[http://www.sciencedirect.com/science/article/pii/S0022030292778382 The least number of phosphate groups for crosslinking of casein by colloidal calcium phosphate] (Aoki et al., 1992) | |||
*[http://www.journalofdairyscience.org/article/S0022-0302%2802%2974097-6 Formation of Reconstituted Casein Micelles with Human β-Caseins and Bovine κ-Casein] (Sood et al., 2002) | |||
*[http://www.ncbi.nlm.nih.gov/pubmed/12906042 Kappa-casein interactions in the suspension of the two major calcium-sensitive human beta-caseins] (Sood et al., 2003) | |||
*[http://link.springer.com/article/10.1007%2Fs10930-005-6715-2 The formation of casein micelles reconstituted with Ca+2 and added inorganic phosphate is influenced by the non-phosphorylated form of human beta-casein.] (Sood et al., 2005) | |||
*: The turbidity (OD 400) and a lack of precipitation as temperature (T) was increased from 4 to 37C, were used as an index of micelle formation. | |||
*[http://link.springer.com/article/10.1007%2Fs10930-005-7591-5 Colloidal calcium phosphate in the reconstituted milk micelle may direct wild-type recombinant human beta-casein to fold like the native protein.] (Sood et al., 2005) | |||
*[http://www.sciencedirect.com/science/article/pii/S0268005X10002808 Re-assembled casein micelles and casein nanoparticles as nano-vehicles for ω-3 polyunsaturated fatty acids] (Zimet et al, 2011) | *[http://www.sciencedirect.com/science/article/pii/S0268005X10002808 Re-assembled casein micelles and casein nanoparticles as nano-vehicles for ω-3 polyunsaturated fatty acids] (Zimet et al, 2011) | ||
*: Particle size measurements were performed by dynamic light scattering (DLS) using a Nicomp™ 380 particle size analyzer (PSS, Santa Barbara CA, USA) at 23 °C, using 0.933 cp for the viscosity of the medium. Mean diameters were calculated by the Gaussian distribution analysis. Particle size distributions were analyzed by the Nicomp™ volume-weighted distribution analysis. A detailed description of the Gaussian and the Nicomp™ analysis methods appears in the supplementary data (appendix 1) of Shapira, Assaraf, and Livney (2010). | |||
Note: None of these articles have been downloaded to seafile yet. | Note: None of these articles have been downloaded to seafile yet. | ||
= Purification of caseins from milk or casein powder = | |||
In order to test micelle formation properties of our recombinant caseins, we may need to combine them with natural caseins. So we also need to know how to purify caseins out of milk or casein powder. | |||
This is the protocol that Cory Tobin has been using for his coagulation tests: | |||
* [https://www.ncbi.nlm.nih.gov/pubmed/1452837 Purification of α<sub>s</sub>-, β-, and κ-caseins by batchwise ion-exchange separation.] ([https://drive.google.com/open?id=1bP2BxLzJrtIYl9UQECmBUZefgFrbOuOX pdf]) (Cayot et al, 1992) | |||
Other papers that seem highly relevant: | |||
* [https://www.ncbi.nlm.nih.gov/pubmed/18501629 Gram scale separation of casein proteins from whole casein on a Source 30Q anion-exchange resin column utilizing fast protein liquid chromatography (FPLC).] (Plank et al, 2008) | |||
* [https://www.cambridge.org/core/journals/journal-of-dairy-research/article/preparativescale-purification-of-bovine-caseins-on-a-cationexchange-resin/11D5C3AFED7C463C97EC7F41E1D91680 Preparative-scale purification of bovine caseins on a cation-exchange resin.] (Leaver and Law, 1992) | |||
* [https://www.journalofdairyscience.org/article/S0022-0302(10)00056-1/fulltext Short communication: separation and quantification of caseins and casein macropeptide using ion-exchange chromatography.] (Holland et al, 2010) | |||
* [https://patents.google.com/patent/US20070104847A1/en Purification of beta casein from milk.] (Patent US20070104847A1) | |||
* [https://patents.google.com/patent/US20040234666A1/en Method of extracting casein fractions from milk and caseinates and production of novel products.] (Patent US20040234666A1) | |||
* [https://patents.google.com/patent/KR101867868B1/en Process for the Isolation of A2 β-casein from goat milk and use for food of the same.] (Patent KR101867868B1) | |||
= Health effects of milk/cheese components = | |||
== Cow's Milk Protein Allergy == | |||
Cow's milk allergies seem to be [http://www.ncbi.nlm.nih.gov/pubmed/8837665 caused primarily by casein], and [http://www.ncbi.nlm.nih.gov/pubmed/9531166 especially the alpha-s1 and beta caseins] that are the major component of cow's milk. Switching to breastfeeding seems to resolve the problems, although in some instances the mother may need to avoid dairy products as well. This seems to suggest that we may be able to avoid allergic reactions by replacing the bovine caseins with the human version, or by humanizing [http://www.ncbi.nlm.nih.gov/pubmed/11174208 specific epitopes on the bovine casein]. | |||
== Cow's Milk Protein Intolerance == | |||
Non immunologically reactions against cow's milk protein are defined as cow’s milk protein intolerance (CMPI). | |||
= Human or bovine? = | |||
There seems to be a distinct sociological "ick" reaction against the idea of making cheese made from human casein proteins, even among those perfectly willing to consider genetically engineered cheese. (Also check out the wikipedia sections on [http://en.wikipedia.org/wiki/Breast_milk#Alternative_uses_for_breast_milk Alternative uses for breast milk] and [http://en.wikipedia.org/wiki/Breast_milk#Extraordinary_consumption Extraordinary consumption].) On one hand, there may be significant health benefits associated with humanizing some or part of the casein proteins (probiotic effects, avoiding cow's milk allergies, etc.) On the other hand, we don't know how human caseins would behave in cheese making, and they may make the concept of an engineered cheese even less acceptable to the general public. | |||
Suggested compromise: | |||
* We do both the human and bovine kappa-casein at least. That should tell us whether the chymosin enzyme functions the same way on both, and whether the human kappa-casein can form micelles with bovine caseins. | |||
* We do a little write-up on the Ethical, Legal, Societal Issues surrounding engineering with human genes and making a consumable from them (human burger, anyone?) That'll give us extra points for the iGEM competition as well. | |||
Another suggested compromise: | |||
* Narwhal casein! | |||
Latest revision as of 02:16, 12 March 2024
Intro
Kappa casein is the protein responsible for milk coagulation into cheese when it is cleaved by the chymosin enzyme in rennet. If we could express this protein in yeast, we could essentially make vegan cheese.
A 2005 paper showed that the C-terminal part of the human kappa casein protein can be overexpressed in S. cerevisiae (baker’s yeast) and Pichia pastoris, and they were able to get it secreted in sufficient quantity that they could run the culture medium on a gel, and see a clear band for the protein. (“This macropeptide has various biological activities and is used as a functional food ingredient as well as a pharmaceutical compound.“ - valuable stuff, not just for vegan cheese!)
Our first task would be to replicate this result - ideally in the BioBricks format. Next would be to express and secrete the full-length protein. If we can get it to express and secrete in sufficient quantity, we should be able to show that you get a single band on the gel for the intact protein, but two bands after cleaving the protein with chymosin.
We can have some people working simultaneously on trying to express the other casein proteins (alpha-s1, alpha-s2, and beta). But since these are hydrophobic, we may not be able to express and secrete them without kappa-casein to hold them in suspension in a casein micelle. We can also attempt to modify the yeast lipid biosynthesis pathways to produce milk fats.
Essential reading
As recommended by Patrik:
- Rennet coagulation of milk
- Mechanisms of Coagulation: The principles, the science and what they mean to cheesemakers
- Production of human caseinomacropeptide in recombinant Saccharomyces cerevisiae and Pichia pastoris (on seafile)
Proteins
Patrik D says: Turns out casein proteins are not quite as insoluble as I had feared, especially if you keep the Ca concentration low enough. They've even been explored as chaperones to get other problematic proteins to fold better. There's also some papers on alpha- and beta-casein expression in E. coli and yeast, and on reconstitution of casein micelles from beta and kappa casein (beta casein is the major component in human milk).
Exon-intron structure of bovine milk proteins:
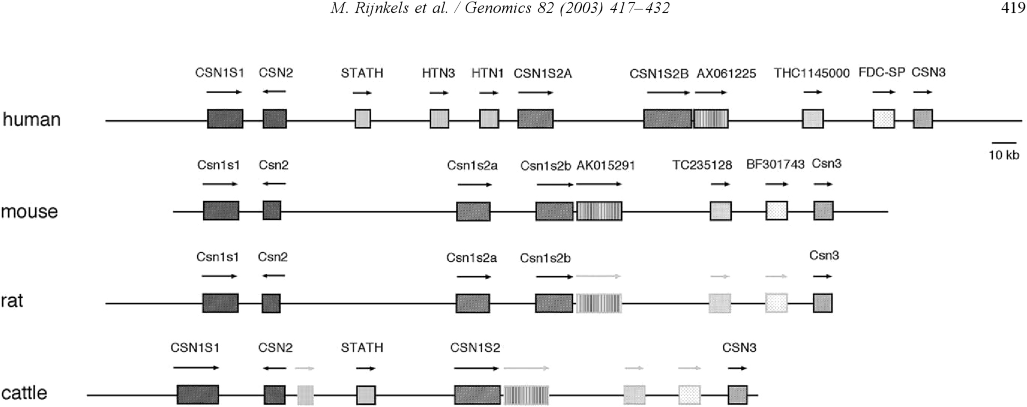
Organization of the casein genes in the human, mouse, rat, and cattle genome:
Milk composition
Mature bovine milk contains (as a percentage of total skim-milk proteins) 39-46% Alpha-s1-casein, 8-11% Alpha-s2-casein, 25-35% Beta-casein, 8-15% Kappa-casein, and 3-7% Gamma-casein (mostly fragments of Beta casein). Caseins make up about 81% of the total protein in bovine milk, but only 30% of the proteins of human milk. The dominant casein in human milk is Beta casein.
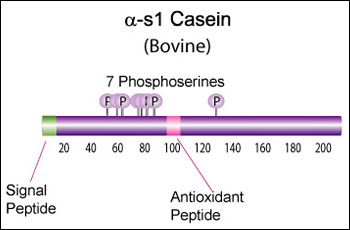
Alpha-s1 casein (CSN1S1)
Major component of cow's milk, along with beta casein.
α-s1 Casein is the most prevalent form of casein in bovine milk. It has been reported to exhibit antioxidant and radical scavenging properties.1 It has also been reported to be involved in the transport of and casein from the endoplasmic reticulum to the Golgi apparatus. [Chanat, E., J. Cell Sci., 112, 3399-3412 (1999)] [1]
Genetic variants & health effects
A recent review of milk protein nomenclature (Farrell et al., 2004) indicated 8 αs1-CN (A, B, C, D, E, F, G, H) [...] variants in the Bos genus. [2]
CSN1S1 C positively affected milk coagulation [3]
Sequences
- Bovine: UniProt P02662 (A variant)
- Human: UniProt P47710
Bovine alpha-S1-casein is a 214 AA protein, including a 15 AA secretion signal. The molecular weight is 24,529 Da.
Alpha-S1 is a key protein in milk protein allergy, and there is some data available on immunoglobulin E (IgE)-binding epitopes (i.e. antigenic peptides) in different genetic variants (Lisson et al., 2014), but there seems to be a lot of variability from patient to patient, and it is not clear (to me at least) what the health effects are of the different variants.
Until we can figure out the health effects, I think we should focus on CSN1S1*C for its improved coagulation properties:
>gi|195973860|gb|ACG63494.1| alpha S1 casein [Bos taurus] MKLLILTCLVAVALARPKHPIKHQGLPQEVLNENLLRFFVAPFPEVFGKEKVNELSKDIGSESTEDQAME DIKQMEAESISSSEEIVPNSVEQKHIQKDDVPSERYLGYLEQLLRLKKYKVPQLEIVPNSAEERLHSMKE GIHAQQKEPMIGVNQELAYFYPELFRQFYQLDAYPSGAWYYVPLGTQYTDAPSFSDIPNPIGSENSGKTT MPLW
Human alpha-S1-casein is present at much lower concentration than in bovine milk, and is only 185 AA, molecular weight 21,671 Da:
>gi|1345671|sp|P47710.1|CASA1_HUMAN RecName: Full=Alpha-S1-casein; Contains: RecName: Full=Casoxin-D; Flags: Precursor MRLLILTCLVAVALARPKLPLRYPERLQNPSESSEPIPLESREEYMNGMNRQRNILREKQTDEIKDTRNE STQNCVVAEPEKMESSISSSSEEMSLSKCAEQFCRLNEYNQLQLQAAHAQEQIRRMNENSHVQVPFQQLN QLAAYPYAVWYYPQIMQYVPFPPFSDISNPTAHENYEKNNVMLQW
Alignment between the bovine reference sequence (A variant), bovine C variant, and human alpha-S1-casein (only 33% sequence identity):
P02662 1 MKLLILTCLVAVALARPKHPIKHQGLPQEVLNENLLRFFVAPFPEVFGKEKVNELS--------------KDIGSESTED 66 ACG63494 1 MKLLILTCLVAVALARPKHPIKHQGLPQEVLNENLLRFFVAPFPEVFGKEKVNELS--------------KDIGSESTED 66 P47710 1 MRLLILTCLVAVALARPKLPLRY---PERLQNPSESS---EPIPLESREEYMNGMNrqrnilrekqtdeiKDTRNESTQN 74 P02662 67 QAMEDIKQMEAESISSSEEIVPNSVEQKHIQKEDVPSERYLGYLEQLLRLKKYKVPQLEIVPNSAEERLHSMKEGIHAQQ 146 ACG63494 67 QAMEDIKQMEAESISSSEEIVPNSVEQKHIQKDDVPSERYLGYLEQLLRLKKYKVPQLEIVPNSAEERLHSMKEGIHAQQ 146 P47710 75 CVVAEPEKMESSISSSSEEMSLSKCA------------------EQFCRLNEYN--QLQLQAAHAQEQIRRMNENSHVQV 134 P02662 147 KEPMIGVNQELAYFYPELFRQFYQLDAYPSGAWYYVPLGTQYTDAPSFSDIPNPIGSENSEKTTMPL-W 214 ACG63494 147 KEPMIGVNQELAYFYPELFRQFYQLDAYPSGAWYYVPLGTQYTDAPSFSDIPNPIGSENSGKTTMPL-W 214 P47710 135 P-----------------FQQLNQLAAYPYAVWYY-PQIMQYVPFPPFSDISNPTAHENYEKNNVMLqW 185
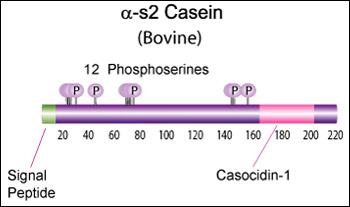
Alpha-s2 casein (CSN1S2)
Genetic variants & health effects
A recent review of milk protein nomenclature (Farrell et al., 2004) indicated [...] 4 αs2-CN (A, B, C, D) [...] variants in the Bos genus. [4]
Proteolytic fragments of α-s2 Casein have been shown to exhibit antibacterial activity. Specifically the 39 amino acid casocidin-1 peptide fragment has been shown to inhibit E. coli and Staph. carnosis growth. [Zucht, H. D., FEBS Lett., 371, 185-188 (1995)] [5]
Sequences
- Bovine: UniProt P02663 (A variant)
Bovine Alpha-s2 casein is a 222 AA protein, including a 15 AA secretion signal peptide. The molecular weight is 26,019 Da.
>sp|P02663|CASA2_BOVIN Alpha-S2-casein OS=Bos taurus GN=CSN1S2 PE=1 SV=2 MKFFIFTCLLAVALAKNTMEHVSSSEESIISQETYKQEKNMAINPSKENLCSTFCKEVVR NANEEEYSIGSSSEESAEVATEEVKITVDDKHYQKALNEINQFYQKFPQYLQYLYQGPIV LNPWDQVKRNAVPITPTLNREQLSTSEENSKKTVDMESTEVFTKKTKLTEEEKNRLNFLK KISQRYQKFALPQYLKTVYQHQKAMKPWIQPKTKVIPYVRYL
In human, the alpha-s2 casein gene is duplicated, but both copies (CSN1S2AP, CSN1S2BP ) are truncated shortly after the signal peptide. Therefore, these loci appear to be a pseudogenes.
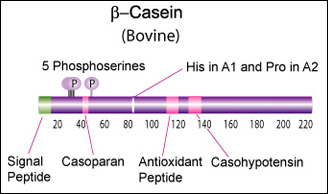
Beta casein (CSN2)
Main component of cow's and human milk.
β-Casein and its fragments have been implicated in a number of biological functions. The casoparan peptide has been reported to activate macrophage phagocytosis and peroxide release. Casohypotensin and casoparan may be involved in bradykinin regulation. Casohypotensin has also been shown to be a strong inhibitor of endo-oligopeptidase A, a thiol-activated protease capable of degrading bradykinin and neurotensin, and hydrolyzing enkephalin-containing peptides to produce enkephalins. β-Caseins are also a source of casomorphin peptides which exhibit opioid activity binding to opioid receptors. Casomorphins may be the hydrolysis product of dipeptidyl peptidase IV. [Miyamoto, Y., et al., Am. J. Physiol. Renal. Physiol., 252, 670-F677 (1987)] [6]
Genetic variants & health effects
A recent review of milk protein nomenclature (Farrell et al., 2004) indicated [...] 12 β-CN (A1, A2, A3, B, C, D, E, F, G, H1, H2, I) [...] variants in the Bos genus. [7]
"CSN2 B positively affected milk coagulation, whereas CSN2 A(2), in particular, had a negative effect" [8]
"Populations, which consume milk containing high levels of β-casein A2 variant, have a lower incidence of cardiovascular disease and type 1 diabetes. Furthermore, consumption of milk with the A2 variant may be associated with less severe symptoms of autism and schizophrenia." [9] "Human milk, goat milk, sheep milk and other species’ milk contain beta-casein which is ‘A2 like’, because they have a proline at the equivalent position in their beta-casein chains." [10].
The health benefits of "A2 milk" (or rather the health risks associated with A1/B beta-casein) are thought to be due to beta-casomorphin 7 (BCM-7), one of a group of degradation peptides with a chain length of 4–11 amino acids (aa), all starting with tyrosine residue in position 60 (Kostyra et al. 2004). The A1 variant (as well as B, C, F, and G) has a histidine rather than Proline in position 67, resulting in more efficient proteolysis at that location by elastase, and a 4-fold higher BCM-7 level in hydrolysed A1 milk vs A2 milk (Kaminski et al, 2007). Human BCM's also show opioid activity (Koch et al., 1985), but may have different health effects due to some amino acid differences.
Sequences
- Bovine: UniProt P02666 (A2 variant)
- Human: UniProt P05814
Bovine beta-casein is a 224 AA protein, including a 15 AA secretion signal. The weight is 25,107 Da. We should try bovine A2 for the reputed health benefits, and B for improved milk coagulation.
A2:
>gi|115660|sp|P02666.2|CASB_BOVIN RecName: Full=Beta-casein; Contains: RecName: Full=Casoparan; Contains: RecName: Full=Antioxidant peptide; Contains: RecName: Full=Casohypotensin; Flags: Precursor MKVLILACLVALALARELEELNVPGEIVESLSSSEESITRINKKIEKFQSEEQQQTEDELQDKIHPFAQT QSLVYPFPGPIPNSLPQNIPPLTQTPVVVPPFLQPEVMGVSKVKEAMAPKHKEMPFPKYPVEPFTESQSL TLTDVENLHLPLPLLQSWMHQPHQPLPPTVMFPPQSVLSLSQSKVLPVPQKAVPYPQRDMPIQAFLLYQE PVLGPVRGPFPIIV
B:
>gi|83406093|gb|AAI11173.1| CSN2 protein [Bos taurus] MKVLILACLVALALARELEELNVPGEIVESLSSSEESITRINKKIEKFQSEEQQQTEDELQDKIHPFAQT QSLVYPFPGPIHNSLPQNIPPLTQTPVVVPPFLQPEVMGVSKVKEAMAPKHKEMPFPKYPVEPFTERQSL TLTDVENLHLPLPLLQSWMHQPHQPLPPTVMFPPQSVLSLSQSKVLPVPQKAVPYPQRDMPIQAFLLYQE PVLGPVRGPFPIIV
Human beta-casein is a 226 AA protein, including a 15 AA secretion signal peptide. The molecular weight is 25,382 Da.
>gi|115661|sp|P05814.4|CASB_HUMAN RecName: Full=Beta-casein; Flags: Precursor MKVLILACLVALALARETIESLSSSEESITEYKQKVEKVKHEDQQQGEDEHQDKIYPSFQPQPLIYPFVE PIPYGFLPQNILPLAQPAVVLPVPQPEIMEVPKAKDTVYTKGRVMPVLKSPTIPFFDPQIPKLTDLENLH LPLPLLQPLMQQVPQPIPQTLALPPQPLWSVPQPKVLPIPQQVVPYPQRAVPVQALLLNQELLLNPTHQI YPVTQPLAPVHNPISV
Alignment between bovine A2, bovine B, and human (55% identity over 72% of the sequence):
P02666 1 MKVLILACLVALALARELEELNVPGEIVESLSSSEESITRINKKIEKFQSEEQQQTEDELQDKIHPFAQTQSLVYPFPGP 80 AAI11173 1 MKVLILACLVALALARELEELNVPGEIVESLSSSEESITRINKKIEKFQSEEQQQTEDELQDKIHPFAQTQSLVYPFPGP 80 P05814 1 MKVLILACLVALALARET---------IESLSSSEESITEYKQKVEKVKHEDQQQGEDEHQDKIYPSFQPQPLIYPFVEP 71 P02666 81 IPNS-LPQNIPPLTQTPVVVPPFLQPEVMGVSKVKEAMAPKHKEMPFPKYPVEPFTESQSLTLTDVENLHLPLPLLQSWM 159 AAI11173 81 IHNS-LPQNIPPLTQTPVVVPPFLQPEVMGVSKVKEAMAPKHKEMPFPKYPVEPFTERQSLTLTDVENLHLPLPLLQSWM 159 P05814 72 IPYGfLPQNILPLAQ-PAVVLPVPQPEIMEVPKAKDTVYTKGRVMPVLKSPTIPFFDPQIPKLTDLENLHLPLPLLQPLM 150 P02666 160 HQPHQPLPPTVMFPPQSVLSLSQSKVLPVPQKAVPYPQRDMPIQAFLLYQEPVLGP------VRGPF-----PIIV 224 AAI11173 160 HQPHQPLPPTVMFPPQSVLSLSQSKVLPVPQKAVPYPQRDMPIQAFLLYQEPVLGP------VRGPF-----PIIV 224 P05814 151 QQVPQPIPQTLALPPQPLWSVPQPKVLPIPQQVVPYPQRAVPVQALLLNQELLLNPthqiypVTQPLapvhnPISV 226
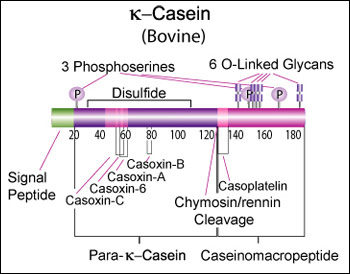
Kappa casein (CSN3)
κ-Casein's orientation on the surface of the casein micelle functions as an interface between the hydrophobic interior caseins and the aqueous environment. During clotting of milk, hydrolysis by chymosin or rennin releases the water soluble fragment, para-k-casein and the hydrophobic caseinomacropeptide. [11]
- Kappa-casein on wikipedia
- Bovine kappa-casein precursor gene CSN3
- kappa-casein genes in other mammals
Genetic variants & health effects
A recent review of milk protein nomenclature (Farrell et al., 2004) indicated [...] 11 κ-CN (A, B, C, E, F1, F2, G1, G2, H, I, J) [...] variants in the Bos genus. [12]
- Casoxins A, B and C have opioid antagonist activity. Casoxin C binds to the complement C3a receptors. Casoplatelin inhibits platelet aggregation. [Lawrence K., Creamer, L. K., et al., Journal of Dairy Science, 81, 3004-3012 (1998)] [13]
- The protein polymorphism at position 148-150 led to 2 different ACE inhibiting peptides. The peptide Val-Ser-Pro derived from CSN3*F1 showed the highest IC50 value (21.8) and is also known from maize (Miyoshi et al., 1991). The peptide Ala-Ser-Pro at the same position of the amino acid sequence but derived from the alleles CSN3*B and CSN3*C is not known for ACE inhibitory effects yet and showed an IC50 value of 242.3.
- The genetic variation at amino acid position 96–99 produced 2 further ACE inhibitory peptides. In CSN3*C, the peptide Ala-His-His-Pro (IC50 = 847.6) was identified and at the same position the peptide Ala-Cys-His-Pro (IC50 = 360.7) was identified in CSN3*G2. These 2 peptides are not known for ACE inhibitory effects yet.
- The dominant genetic variant of CSN3 in German cattle breeds is CSN3*A, whereas the B allele shows a high frequency in German Brown (0.479; Erhardt, 1989). Compared with CSN3*A, the B allele shows association with higher milk protein content and positive effects on coagulation properties and cheese yield (NgKwai-Hang and Grosclaude, 1992). In addition to these positive attributes, the present study shows another reason for preferring milk with the κ-casein allele B, because of the appearance of a new ACE inhibitory peptide at position 148–150 of the protein. [14]
- CSN3 B positively affected milk coagulation [15]
Caseinomacropeptide
Kim YJ, Oh YK, Kang W, Lee EY, Park S. Production of human caseinomacropeptide in recombinant Saccharomyces cerevisiae and Pichia pastoris. J Ind Microbiol Biotechnol. 2005 Sep;32(9):402-8.
“Caseinomacropeptide is a polypeptide of 64 amino acid residues (106-169) derived from the C-terminal part of the mammalian milk k-casein. This macropeptide has various biological activities and is used as a functional food ingredient as well as a pharmaceutical compound. The gene encoding the human caseinomacropeptide (hCMP) was synthesized and expressed with an alpha-factor secretion signal in the two yeast strains, Saccharomyces cerevisiae and Pichia pastoris. The complete polypeptide of the recombinant hCMP was produced and secreted in a culture medium by both the strains, but the highest production was observed in S. cerevisiae with a galactose-inducible promoter. In a fed-batch bioreactor culture, 2.5 g/l of the recombinant hCMP was obtained from the S. cerevisiae at 97 h.” “Escherichia coli XL1-Blue (Stratagene, USA) was used for cloning and propagating genes. Host strains for recombinant hCMP were S. cerevisiae 2805 (his-, ura-) [...] For S. cerevisiae, pYIGP (containing a constitutive GAP promoter) or pYEGa (containing a galactose-inducible GAL promoter) was used.”
Sequence
- Bovine: UniProt P02668
- Human: UniProt P07498
Bovine kappa-casein is 190 AA of which the first 21 AA form a secretion signal. The molecular weight is 21,269 Da. The bovine reference sequence is Genbank AY380228, RefSeq NP_776719, corresponding to the CSN*A allele.
Because of improved coagulation time, and possible additional health benefits, we should focus on bovine genetic variant CSN3*B instead:
>gi|36988716|gb|AAQ87923.1| kappa casein [Bos taurus] MMKSFFLVVTILALTLPFLGAQEQNQEQPIRCEKDERFFSDKIAKYIPIQYVLSRYPSYGLNYYQQKPVA LINNQFLPYPYYAKPAAVRSPAQILQWQVLSNTVPAKSCQAQPTTMARHPHPHLSFMAIPPKKNQDKTEI PTINTIASGEPTSTPTIEAVESTVATLEASPEVIESPPEINTVQVTSTAV
(secretion signal in bold; caseinomacropeptide in italic)
Human kappa-casein is 182 AA. The molecular weight is 20,305. The main human variant is:
>gi|1705606|sp|P07498.3|CASK_HUMAN RecName: Full=Kappa-casein; Flags: Precursor MKSFLLVVNALALTLPFLAVEVQNQKQPACHENDERPFYQKTAPYVPMYYVPNSYPYYGTNLYQRRPAIA INNPYVPRTYYANPAVVRPHAQIPQRQYLPNSHPPTVVRRPNLHPSFIAIPPKKIQDKIIIPTINTIATV EPTPAPATEPTVDSVVTPEAFSESIITSTPETTTVAVTPPTA
Alignment between bovine A, bovine B, and human (53% sequence identity):
P02668 1 MMKSFFLVVTILALTLPFLGAQEQNQEQPIRCEKDERFFSDKIAKYIPIQYVLSRYPSYGLNYYQQKPVALINNQFLPYP 80 AAQ87923 1 MMKSFFLVVTILALTLPFLGAQEQNQEQPIRCEKDERFFSDKIAKYIPIQYVLSRYPSYGLNYYQQKPVALINNQFLPYP 80 P07498 1 -MKSFLLVVNALALTLPFLAVEVQNQKQPACHENDERPFYQKTAPYVPMYYVPNSYPYYGTNLYQRRPAIAINNPYVPRT 79 P02668 81 YYAKPAAVRSPAQILQWQVLSNTVPAKSCQAQPTTMARHPHPHLSFMAIPPKKNQDKTEIPTINTIASGEPTSTPTTEAV 160 AAQ87923 81 YYAKPAAVRSPAQILQWQVLSNTVPAKSCQAQPTTMARHPHPHLSFMAIPPKKNQDKTEIPTINTIASGEPTSTPTIEAV 160 P07498 80 YYANPAVVRPHAQIPQRQYLPN--------SHPPTVVRRPNLHPSFIAIPPKKIQDKIIIPTINTIATVEPTPAPATEPT 151 P02668 161 ESTVATLEDSPEVIE-SPPEINTVQVTSTAV 190 AAQ87923 161 ESTVATLEASPEVIE-SPPEINTVQVTSTAV 190 P07498 152 VDSVVTPEAFSESIItSTPETTTVAVTPPTA 182
Post-translational modification
According to the Uniprot record, the post-translation modifications include:
- Glycosylation of six residues, all of them Threonine ((O-GalNAc)
- Phosphorylation of three residues, all of them Serine
- One disulphide bond between two cysteine residues, potentially important for multimerization [16]
- One "Pyrrolidone carboxylic acid" modification to a glutamine (?)
We should investigate if any of this is important for micelle formation or micellar flocculation.
Quote from University of Illinois' Milk Composition & Synthesis Resource Library:
Phosphorylation is a ubiquitous biological process. It is involved in many regulatory mechanisms. It occurs after completion of the polypeptide chain. It can occur at Ser, Thr, Glu, Asp, His, Lys, and Tyr. Many enzymes will phosphorylate proteins with ATP, but they differ in specificity. These are called Kinases. Kinases are membrane-bound in the smooth endoplasmic reticulum and Golgi.
Caseins are phosphoproteins.
- k-Casein is not extensively phosphorylated- probably because of competition between the glycosyltransferases and the kinases for sites on the protein.
- Caseins are in multiphosphorylated forms - the same casein polypeptide chain may be phosphorylated at varying sites.
- Probably due to the tremendously high rate of protein synthesis which exceeds the phosphorylating capacity of the membrane-bound kinases.
Role of phosphorylation and glycosylation in micelle formation or coagulation
See our separate page on Phosphorylation and glycosylation of caseins for a more in-depth look at the literature.
2-Dimensional electrophoresis (2-DE) can be used to distinguish κ-CN isoforms. In a study of milk from Holstein-Friesian and Jersey cows, about 1/3 of kappa-casein was glycosylated, and almost all phosphorylated. The dominant isoform was 1P (40-50%), with 2P, 1P+1G, 1P+2G and 1P+3G each around 10-15%. [17]
- studies on the relationship between κ-CN glycosylation and MCP [Milk Coagulation Properties] provide inconsistent results. Cases et al. (2003) estimated a favorable effect of κ-CN deglycosylation on acid coagulation of milk, with deglycosylated milk exhibiting gel formation at greater pH and increased gel stiffness compared with untreated milk. According to Robitaille et al. (1993) and Mariani et al. (1995), κ-CN glycosylation favorably affects both the rate of firming and the firmness of the curd, but its effects on coagulation time are trivial. Jensen et al. (2012b) described favorable effects of the glycosylated κ-CN fraction on rennet coagulation but, in a later study ( Jensen et al., 2012a), detected no significant difference in the κ-CN posttranslational modification pattern between samples exhibiting good or poor coagulation properties. [...]
- Results from this study indicate that variation in RCT [Rennet Coagulation Time] attributable to κ-CNtot must be entirely ascribed to the glycosylated component of the protein. [...]
- The degree of κ-CN glycosylation is a key determinant of protein hydrophobicity (O’Connell and Fox, 2000) [...] From a theoretical perspective, an increased degree of glycosylation is thus expected to stabilize the micelle structure (Dziuba and Minkiewicz, 1996) and to increase the time needed by chymosin to activate milk coagulation. However, there is no conclusive evidence that κ-CN is a key element for stability of the native caseinate emulsions [...] [18]
Casein Kinase
There are some confusing factors when talking about casein kinase. There are enzymes in most eukaryotes called Casein Kinase 1 and 2. These apparently don't have much to do with casein:
The name casein kinase 2 arose from the fact that casein is a convenient protein substrate that is generally used to assay its activity ... many researchers that work with this enzyme agreed to abandon the reference to casein because it leads to confusion about a possible physiological role for this substrate. The name protein kinase CK2 was recommended. -- allende 1995
In fact, until 2012 the kinases responsible for casein phosphorylation were unknown. The actual kinase is a secreted kinase called Fam20C (Tagliabracci et al. 2012).
Fam20C specifically recognizes S-x-E/pS (where x is any amino acid and E/pS can be Glu or phosphoserine).
Gene
The gene is also known as DMP4. The human version is 584 AA long (including the signal peptide). The molecular weight is 66,234 Da.
Required minerals/ions
Fam20C phosphorylated ... [one of the sites phosphorylated in b-casein] ... in the presence of MnCl2 and CoCl2 more efficiently than in the presence of MgCl2 and CaCl2.
Relevant articles
Older notes and more info
Two species of casein kinase from lactating bovine mammary gland have been identified; a Ca2+- and CM-independent casein kinase and a Ca2+- and CM-dependent casein kinase. The Ca2+- and CM-independent casein kinase phosphorylates previously dephosphorylated αs1-, β- or κ-casein while the Ca2+- and CM-dependent casein kinase prefers previously dephosphorylated β- or κ-casein as substrates. Two activities are indicated by their substrate specificity, sensitivity to Ca2+ and CM, pH maxima, and differential solubilization by anionic detergents. The presence of a regulated casein kinase in the lactating mammary gland suggests that casein phosphorylation may be a regulator of micelle formation or secretion.[19]
Fam20C appears to be the Golgi casein kinase that phosphorylates secretory pathway proteins within S-x-E motifs. Fam20C phosphorylates the caseins and several secreted proteins implicated in biomineralization
[Casein kinase-1 and casein kinase-2] are physiologically unrelated to casein because they are mainly cytosolic and nuclear proteins that would be unlikely to encounter casein in the secretory pathway and have therefore been renamed protein kinase CK1 and protein kinase CK2. A physiological casein kinase activity has been characterized from highly enriched Golgi fractions of lactating mammary gland, liver, brain, and kidney and named Golgi-enriched fraction casein kinase (GEF-CK). The GEF-CK specifically recognizes the consensus S-x-E/pS (where x is any amino acid and E/pS can be Glu or phosphoserine). [...] A peptide substrate reproducing one of the sites phosphorylated in β-casein, KKIEKFQSE-EQQQ (β28-40), is selectively phosphorylated by GEF-CK. [...] Protein immunoblotting of immunoprecipitates from conditioned medium of U2OS cells overexpressing V5-tagged αs1-casein with FLAG-tagged Fam20C revealed a mobility shift in αs1-casein that was absent when αs1-casein was coexpressed with the inactive Fam20C D478A mutant [...] Thus, Fam20C appears to phosphorylate casein in vitro and in mammalian cells. -- source
Woah. That changes things. The uniprot record for alpha-s1 shows that all phosphorylated residues except one follow the S-x-E/pS pattern. If this is true for the other casein proteins, then this Fam20C is likely what we want, and not casein kinase II. (Juul (talk))
Beta-casein expressed in E. coli has been successfully phosphorylated by co-expression of casein kinase II: Expression and characterization of phosphorylated recombinant human beta-casein in Escherichia coli. We do not know if CK2 will also work correctly for alpha-casein (for which phosphorylation is important in micelle formation) nor for kappa-casein (where phosphorylation is probably less important), but there is probably a good chance that it does. More research needed.
S. cerevisiae does have Casein Kinase II, but it is somewhat different from bovine CK2. Here's a comparison:
- Casein kinase II subunit alpha: yeast vs. cow: ~49% identity
- Casein kinase II subunit alpha': yeast vs. cow: ~54% identity
- Casein kinase II subunit beta: yeast vs. cow: ~32% identity
- Casein kinase II subunit beta': yeast vs. cow: ~38% identity
- Cow does not have a beta' so this last comparison is between yeast beta' and cow beta.
Is there any way we can gauge if the yeast CK2 is likely to work? If we co-express bovine CK2, how might that affect the yeast and the casein?
Sequence
- Bovine: UniProt F1MXQ3 (unreviewed)
- Human: UniProt Q8IXL6
The Human FAM20C protein is 584 AA, including a 22 AA signal peptide:
>gi|327478506|sp|Q8IXL6.2|DMP4_HUMAN RecName: Full=Extracellular serine/threonine protein kinase FAM20C; AltName: Full=Dentin matrix protein 4; Short=DMP-4; AltName: Full=Golgi-enriched fraction casein kinase; Short=GEF-CK; AltName: Full=Protein FAM20C; Flags: Precursor MKMMLVRRFRVLILMVFLVACALHIALDLLPRLERRGARPSGEPGCSCAQPAAEVAAPGWAQVRGRPGEP PAASSAAGDAGWPNKHTLRILQDFSSDPSSNLSSHSLEKLPPAAEPAERALRGRDPGALRPHDPAHRPLL RDPGPRRSESPPGPGGDASLLARLFEHPLYRVAVPPLTEEDVLFNVNSDTRLSPKAAENPDWPHAGAEGA EFLSPGEAAVDSYPNWLKFHIGINRYELYSRHNPAIEALLHDLSSQRITSVAMKSGGTQLKLIMTFQNYG QALFKPMKQTREQETPPDFFYFSDYERHNAEIAAFHLDRILDFRRVPPVAGRMVNMTKEIRDVTRDKKLW RTFFISPANNICFYGECSYYCSTEHALCGKPDQIEGSLAAFLPDLSLAKRKTWRNPWRRSYHKRKKAEWE VDPDYCEEVKQTPPYDSSHRILDVMDMTIFDFLMGNMDRHHYETFEKFGNETFIIHLDNGRGFGKYSHDE LSILVPLQQCCRIRKSTYLRLQLLAKEEYKLSLLMAESLRGDQVAPVLYQPHLEALDRRLRVVLKAVRDC VERNGLHSVVDDDLDTEHRAASAR
Genetic variants
Excellent review: Milk protein polymorphisms in cattle: Effect on animal breeding and human nutrition. [20]
"Genetic variants can result from SNP, as well as nucleotide (nt) deletions or insertions. A recent review of milk protein nomenclature (Farrell et al., 2004) indicated 8 αs1-CN (A, B, C, D, E, F, G, H), 4 αs2-CN (A, B, C, D), 12 β-CN (A1, A2, A3, B, C, D, E, F, G, H1, H2, I), 11 κ-CN (A, B, C, E, F1, F2, G1, G2, H, I, J), 11 β-LG (A, B, C, D, E, F, G, H, I, J, W), and 3 α-LA (A, B, C) variants in the Bos genus."
"Substantial variation in milk coagulation properties has been observed among dairy cows. [...] the cows were genotyped for major genetic variants in the αS1- (CSN1S1), β- (CSN2), and κ-casein (CSN3) genes, revealing distinct differences in variant frequencies among breeds. [...] CSN1S1 C, CSN2 B, and CSN3 B positively affected milk coagulation, whereas CSN2 A(2), in particular, had a negative effect" [21]
Host organism
Which organism should we use? We've mostly assumed we were going with S. cerevisiae but Pichia pastoris might be preferable. Here is a review comparing the two.
P. pastoris
Advantages over S. cerevisiae
- Likely to have fewer problems with efficient protein secretion
- It can grow to very high cell densities
- It can grow on methanol which is cheap
- This is kinda funny, since we can make methanol from methane (cow-farts) which means that our organism can eat the major greenhouse gas produced by cows.
"Pichia can grow to very high cell densities, and under ideal conditions can multiply to the point where the cell suspension is practically a paste. As the protein yield from expression in a microbe is roughly equal to the product of the protein produced per cell and the number of cells, this makes Pichia of great use when trying to produce large quantities of protein without expensive equipment" -- wikipedia quoting Cregg et al. "Expression in the yeast Pichia pastoris" 2009
Potential problems
- It is unclear if it is safe for human consumption (meaning more purification needed)
- Fewer resources available
S. cerevisiae
Advantages
- Familiar as a human consumable
- FDA GRAS status
- We can probably get away with less or no purification and still have a food-safe product
Potention problems
"Although S. cerevisiae has a well-developed eukaryotic secretory pathway, it is not the most efficient yeast for high-level export of proteins to the extracellular medium."
"...often transport to the extracellular medium is inefficient, especially for complex mammalian proteins of high molecular weight."
-- Overview of Protein Expression in Saccharomyces cerevisiae by Strausberg and Strausberg
Strain selection
The Johns Hopkins' team used YPH500
- List of commonly used lab strains
- A comparison of four strains (article access?)
Carolina yeast sensitive yeast strain
ATCC protease negative strain:
Vectors
The Jons Hopkins iGEM team modified pRS400-series vectors, removing the MCS and replacing them with biobrick prefix and suffix.
Someone (not Patrik) said that galactose induction is the most common form of controlling heterologous expression. Common vectors are the pYES2 and pESC series vectors.
Secretion
Secretion of proteins will require a secretion signal (triggering processing through the ER, golgi and then exocytosis).
BioBricks Parts Registry
- VitaYeast project, by Johns Hopkins’ 2011 iGEM team
- Characterized promoters and terminators
- Yeast parts in the BioBricks parts registry
Plasmid backbones
The BioBricks parts registry contains 17 plasmid backbones, including 9 listed as "Available and Working".
Most of these plasmid backbones are based on pRS315 or pRS305 comply with the AarI part assembly standard developed by the Lim lab. AarI is a rare 7-cutter Type IIS restriction enzyme that cuts 4bp offset from its binding site, and can be used for scarless ligation of up to 3 parts (+ backbone) simultaneously. For more information, see:
Promoters
The BioBricks parts registry contains 44 yeast promoters, including:
Other notes
pESC contains a bidirectional promoter which may allow expression of both parts of the protein without cleavage being required. How?
Published casein expression papers
Is there a common thread in published casein expression work? Do they use similar secretion signals, promoters, etc?
Kim et al., 2005: Production of human caseinomacropeptide in recombinant Saccharomyces cerevisiae and Pichia pastoris
The 64 AA (106-169) caseinomacropeptide of human kappa-casein was expressed and secreted at 120mg/L in S. cerevisiae from an inducible promoter. Expression from consitutive promoter, and expression in Pichia pastoris resulted in lower amounts of secreted protein.
Yeast: S. cerevisiae 2805 (MATα pep4::HIS3 pro1-d can1 GAL2 his3 d ura3–52)
Plasmid: pYEGα (Inducible, GAL 10 promoter, URA 3 marker, ampicillin resistance)
pYEGα is based on YEp352, a multicopy number yeast-E.coli shuttle vector containing pBR322 sequence, yeast URA3 and yeast 2u replication origin. (Sohn et al., 1991)
"The gene for the α-factor secretion signal sequence was placed before the hCMP structural gene so that the signal peptide was fused to the 5' end of the hCMP and that the recombinant hCMP was secreted in the culture medium. In addition, the Kozak sequence was inserted into the position preceding the ATG initiation codon to facilitate an efficient translation in yeast." (see also Figure 2 in Sohn et al., 1991)
Estimating amount of expressed protein
In the 2005 Kim et al. paper where they express caseinomacropeptide they describe using densitometry to estimate amount of expressed protein. They run the stained protein on an SDS-PAGE and use a gel imager to estimate amount of protein. There is an interesting article on how to use a normal scanner for densitometry here. We'd need at least of couple of calibration points which could be known amounts of a different protein. We'd have to know if the stain shows in equal amounts for the different proteins (per mole or per gram).
Casein micelles and cheese curd coagulation
Casein micelles are an essential component of milk, and cheese making. Curd formation is due to coagulation of casein micelles into a 3D network (a gel) that then traps any fat globules as well. So, no micelles - no cheese!
- Kinetics of milk coagulation: II. Kinetics of the secondary phase: micelle flocculation. (Carlson et al, 1987)
- Casein Interactions: Casting Light on the Black Boxes, the Structure in Dairy Products (Home, 1998, review)
- Studies of casein micelle structure: the past and the present (Qi, 2007, review)
If the model proposed by Home is correct, then micelle formation will require alpha, beta, kappa-casein and colloidal calcium. The model also implies that the phosphoserine residues of both alpha and kappa-casein play an important role in micelle formation.
Various groups have resynthesized casein micelles from purified beta and kappa casein
Synthetic casein micelles
Some researchers have created synthetic casein micelles. We should study their efforts:
- Sub-structure of synthetic casein micelles (Knoop et al, 1979)
- A phosphate-induced sub-micelle-micelle equilibrium in reconstituted casein micelle systems (Slattery, 1979)
- The least number of phosphate groups for crosslinking of casein by colloidal calcium phosphate (Aoki et al., 1992)
- Formation of Reconstituted Casein Micelles with Human β-Caseins and Bovine κ-Casein (Sood et al., 2002)
- Kappa-casein interactions in the suspension of the two major calcium-sensitive human beta-caseins (Sood et al., 2003)
- The formation of casein micelles reconstituted with Ca+2 and added inorganic phosphate is influenced by the non-phosphorylated form of human beta-casein. (Sood et al., 2005)
- The turbidity (OD 400) and a lack of precipitation as temperature (T) was increased from 4 to 37C, were used as an index of micelle formation.
- Colloidal calcium phosphate in the reconstituted milk micelle may direct wild-type recombinant human beta-casein to fold like the native protein. (Sood et al., 2005)
- Re-assembled casein micelles and casein nanoparticles as nano-vehicles for ω-3 polyunsaturated fatty acids (Zimet et al, 2011)
- Particle size measurements were performed by dynamic light scattering (DLS) using a Nicomp™ 380 particle size analyzer (PSS, Santa Barbara CA, USA) at 23 °C, using 0.933 cp for the viscosity of the medium. Mean diameters were calculated by the Gaussian distribution analysis. Particle size distributions were analyzed by the Nicomp™ volume-weighted distribution analysis. A detailed description of the Gaussian and the Nicomp™ analysis methods appears in the supplementary data (appendix 1) of Shapira, Assaraf, and Livney (2010).
Note: None of these articles have been downloaded to seafile yet.
Purification of caseins from milk or casein powder
In order to test micelle formation properties of our recombinant caseins, we may need to combine them with natural caseins. So we also need to know how to purify caseins out of milk or casein powder.
This is the protocol that Cory Tobin has been using for his coagulation tests:
- Purification of αs-, β-, and κ-caseins by batchwise ion-exchange separation. (pdf) (Cayot et al, 1992)
Other papers that seem highly relevant:
- Gram scale separation of casein proteins from whole casein on a Source 30Q anion-exchange resin column utilizing fast protein liquid chromatography (FPLC). (Plank et al, 2008)
- Preparative-scale purification of bovine caseins on a cation-exchange resin. (Leaver and Law, 1992)
- Short communication: separation and quantification of caseins and casein macropeptide using ion-exchange chromatography. (Holland et al, 2010)
- Purification of beta casein from milk. (Patent US20070104847A1)
- Method of extracting casein fractions from milk and caseinates and production of novel products. (Patent US20040234666A1)
- Process for the Isolation of A2 β-casein from goat milk and use for food of the same. (Patent KR101867868B1)
Health effects of milk/cheese components
Cow's Milk Protein Allergy
Cow's milk allergies seem to be caused primarily by casein, and especially the alpha-s1 and beta caseins that are the major component of cow's milk. Switching to breastfeeding seems to resolve the problems, although in some instances the mother may need to avoid dairy products as well. This seems to suggest that we may be able to avoid allergic reactions by replacing the bovine caseins with the human version, or by humanizing specific epitopes on the bovine casein.
Cow's Milk Protein Intolerance
Non immunologically reactions against cow's milk protein are defined as cow’s milk protein intolerance (CMPI).
Human or bovine?
There seems to be a distinct sociological "ick" reaction against the idea of making cheese made from human casein proteins, even among those perfectly willing to consider genetically engineered cheese. (Also check out the wikipedia sections on Alternative uses for breast milk and Extraordinary consumption.) On one hand, there may be significant health benefits associated with humanizing some or part of the casein proteins (probiotic effects, avoiding cow's milk allergies, etc.) On the other hand, we don't know how human caseins would behave in cheese making, and they may make the concept of an engineered cheese even less acceptable to the general public.
Suggested compromise:
- We do both the human and bovine kappa-casein at least. That should tell us whether the chymosin enzyme functions the same way on both, and whether the human kappa-casein can form micelles with bovine caseins.
- We do a little write-up on the Ethical, Legal, Societal Issues surrounding engineering with human genes and making a consumable from them (human burger, anyone?) That'll give us extra points for the iGEM competition as well.
Another suggested compromise:
- Narwhal casein!