SDS Page-Gel for all the bovine casein inserts
17 January 2015: SDS Page-Gel All 5 Bovine Casein Inserts
Location: BioCurious
Participants: Lafia, Johan, Meenakshi, Emi, Rachel
Notes: Emi & Rachel
Aims: Confirmation of bovine casein production from liquid culture supernatant of transformed yeast cells
Materials
Gel loading tips (BioRad)
Tris-Glycine SDS Running Buffer 10X stock (Novex Life Tech, Catalog number: LC2675)
Tris-Glycine Mini Gels 4-20% 1.0mm x 10 wells/gel, (Catalog number: EC6025Box, Lot 14082110, received September 2014, possibly stored at -20C for a period of time)
Protein ladder (10-250 kDa) (NEB, Catalog number: p7703S)
6X loading dye + SDS solution (Fermentas)
Micellular casein powder from CCL stock (for positive control)
Chymosin solution. Chymosin cleaves kappa casein into two peptides, allowing us a positive ID of this protein band (CHY-MAX, CCL stock)
Gel box and power supply from BioCurious
Simply Blue Safe Stain LC6060 Lot#1540663
Source cultures
--> Running supernatants of yeast transformations of all 5 bovine casein inserts. Transformation = Yeast Transformation of All Bovine Casein Inserts. Positive colonies consistent in appearance with yeast on the no-plasmid, negative control plate! Multiple positive colonies from each plate (including negative control plates) inoculated to XX medium, 15Jan2015, and grown at 30C in the top of the double-stack incubator in 50mL conical tubes with gentle shaking. Constructs:
1) Bov alpha s1 (1) Transformed liquid culture
2) Bov beta B (8) Transformed liquid culture
3) Bov alpha S2 (3) Transformed liquid culture
4) Bov beta A2 (6B) Transformed liquid culture
5) Bov kappa (7F) Transformed liquid culture
6) Transformed negative control
Protocol
To pellet yeast cells: centrifuge the falcon tubes for 5 minutes 2800xg.
- Preparation of casein control
- Take 500ul of distilled water in eppendorf tube + few grains of casein powder (use 200ul hp). Vortex vigorously.
- Divide in two 2.0mL microfuge tubes, 2 250ul aliquots, 1 to be treated with chymosin, 1 not.
- Take 500ul of distilled water in eppendorf tube + few grains of casein powder (use 200ul hp). Vortex vigorously.
- Chymosin treatments:
- Added 15ul chymosin solution (Should be end 1nM in literature, didn't know the concentration of CHY-MAX, saw suggestion 1:5-15 in CHY-MAX cheese recipes) to 250ul of:
- 1) casein solution, above,
- 2) diH20 (chymosin-only control)
- 3) bovine kappa casein (7F) supernatant
- Incubated 20min at 32C for casein-cleavage reaction.
- Added 15ul chymosin solution (Should be end 1nM in literature, didn't know the concentration of CHY-MAX, saw suggestion 1:5-15 in CHY-MAX cheese recipes) to 250ul of:
- Preparation of samples to load on gel
- Take 40ul of 1, 8, 3B, 6B, +7F also the negative control to eppendorf tubes
- 5ul of 6x loading dye to each tube (this isn't to final conc. of 1x; should be 8ul)
- Boil all the tubes for 10 mins at 95C (in order to denature the protein structure to linear)
- Loading & Running Gel Generally, followed manufacturer's protocol for running Tris-Glycine 4%-20% mini-gels, Media:LifeTech_NuPAGE_TrisGlycine_Mini_Gels_Cat-EC6025BOX.pdf
- Preparation of 20x running buffer = Pour 50ml of 10x Tris-Glycine running buffer; bring to 500mL with 450mL diH2O
- Assembled gel box with inner chamber walls = two sample gels
- Filled buffer in inner chamber to cover wells, in outer chamber, to cover gel window.
Gel #1
| Lane | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| bov alpha S1(1) | bov alpha S2(3B) | bov beta A2(6B) | bov kappa(7F) | bov beta B(8) | yellow contam. | (-)control colonies | 10ul ladder | Casein | Casein + chymosin | |
Gel #2
| Lane | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
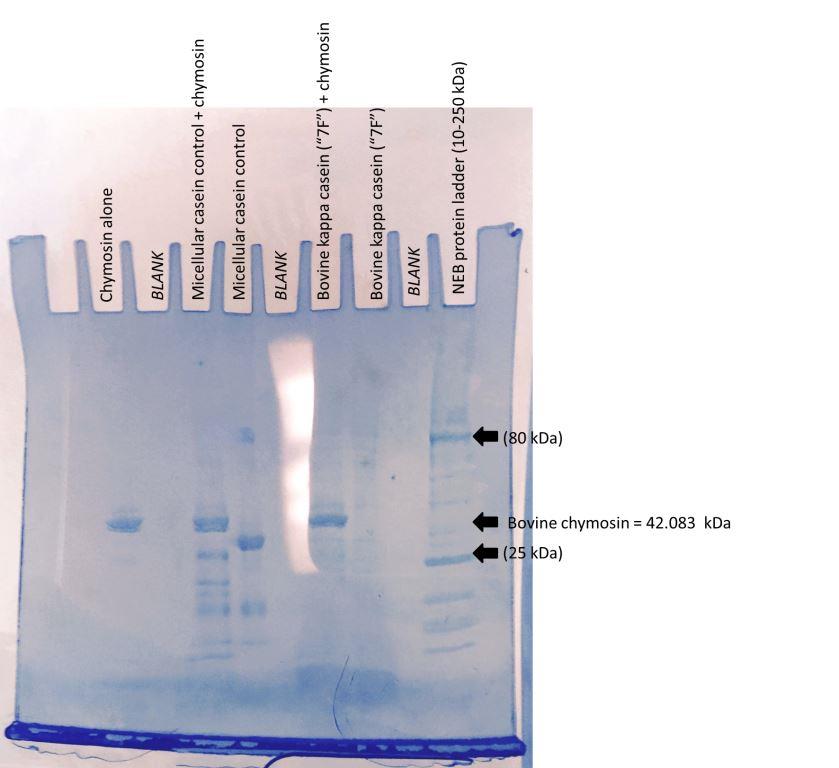
| 10uL Ladder | X | bov kappa(7F) | bov kappa(7F) + chymosin | X | casein | casein + chymosin | X | chymosin-alone control | X | |
- Loaded 30uL of each sample except Gel #1, bovine alpha S1, for which loaded 40uL (vol. too high). As casein has strong bands, didn't load casein (+) next to sample wells.
- Ran at 125 V.
- Gel #1 appeared to have crack and samples started leaking out of crack! To preserve remaining samples, stopped and stained this gel after 45 min. Re-made inner chamber with gel spacer, continued running Gel #2 for full 90 min.
- Staining and destaining gels
- 1) Rinse with diH2O 3x, 5 min each with gentle shaking. Discard each rinse.
- 2) Stain with Simply Blue Safe Stain 1 hr, RT, with gentle shaking. Discard stain.
- 3) Destain in H2O 1hr, gentle shaking. Can repeat if need clear gel background.
Results
Gel#2
Discussion & Next Steps Gel probably cracked because was over 8-week shelf life and had been frozen. Can see chymosin band. Chymosin cleaved micellular caseins.