Difference between revisions of "Cheese making/Experiment 3"
| Line 39: | Line 39: | ||
[[File:RennetUsed.jpg|thumb|The vegetable rennet that was used.]] | [[File:RennetUsed.jpg|thumb|The vegetable rennet that was used.]] | ||
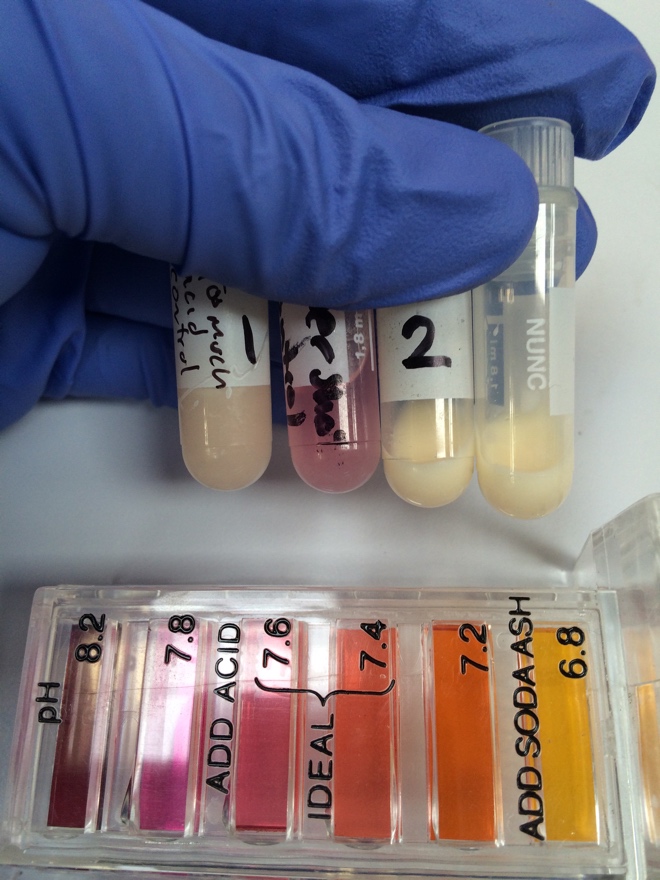
[[File:MilkpHtest.jpg|thumb|Vial # 2 contains the milk before the addition of culture, the unlabeled vial contains the milk 1/2hr after addition of the culture. The vial on the far left is re-milk with acid added so that it would intentionally overshoot the range of the pH scale.]] | |||
[[File:MilkCurds1.jpg|thumb|The consistency of the milk curds.]] | |||
Materials | Materials | ||
| Line 149: | Line 152: | ||
The pH was then adjusted down to about 6.8 with addition of 1:100 solution of HCl, and the culture and rennet were again added, following the above described procedure. After the 30 minute incubation and 40 minute rennet reaction, again no noticeable curd formation could be detected. | The pH was then adjusted down to about 6.8 with addition of 1:100 solution of HCl, and the culture and rennet were again added, following the above described procedure. After the 30 minute incubation and 40 minute rennet reaction, again no noticeable curd formation could be detected. | ||
'''Testing of the culture and rennet''' | |||
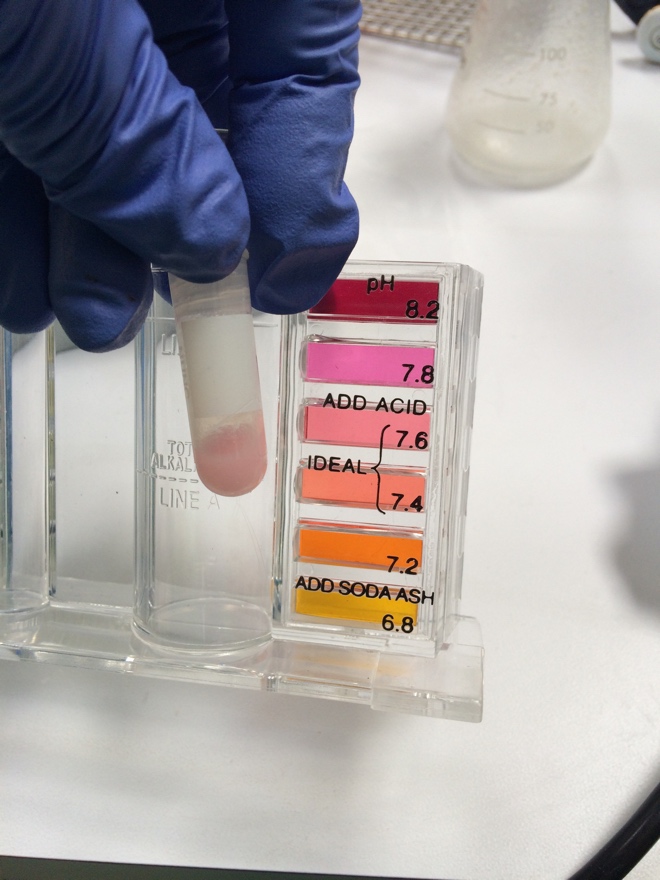
The culture and rennet was tested on real whole milk from Trader Joes. In short, 125mL of milk was heated to 30 degrees C. The pH was tested and was found to be more acidic than the pH test kit's lowest range (see photo). 50mg of mesophilic culture was then added, allowed to absorb in for 2 minutes, then mixed into the warm milk. After 30 minutes, the pH was measured again, showing no apparent change. The vegetable rennet was then added and mixed in for 2 minutes. This mixture was then allowed to sit at 30 degrees C for 40 minutes. | |||
At then end of this time, curd was observed -- the milk had congealed to a consistency similar to Greek yogurt. This was taken as evidence that the rennet was still viable. The curd was placed in the fridge and cheese | |||
'''Discussion''' | '''Discussion''' | ||
Revision as of 02:19, 13 March 2015
Goal
Test production of cheese from re-assembled milk. Re-assembled milk is to be made from
- micellar casein powder
- lactose
- salts
- ghee
Background
Production of cheese requires fats, sugars and salts as well as the casein protein. Our molecular biology team is currently developing a yeast-based casein production platform, but to achieve a product that is completely vegan the sugars and fats must come from non-animal sources as well. The goal of this series of experiments is to test the use of alternate sugars for the cheese ripening process.
The purpose of Experiment 3 in particular is to test an initial positive control: production of cheese from re-assembled milk. This re-assembled milk is produced by combining micellar casein powder, lactose, ghee and a mixture of salts. This expands on the casein solubilization techniques developed in Experiment 2, adding sugars, oils and salts, then ultimately producing cheese from the re-assembled milk.
Methods and results
Materials
- Micellar casein powder: "BodyTech 100% micellar casein."
- Ghee: from Safeway
- Lactose: from Real Vegan Cheese team supplies box.
- Salts: TSP from Home Depot, CaCl2 from Oak Barrel wine making supply store, Mg2+ pill form from Vitamin Shoppe.
- Cheese culture: mesophilic, from Oak Barrel wine making supply store.
- Vegetable rennet: from Oak Barrel wine making supply store.
Re-milk creation
A number of websites were surveyed, finding a range of concentrations for milk's primary components: protein, lactose and lipids. The results from this survey are found in the following table.
| units | water | protein | lactose | fat | source |
| percent of solid content | 3.5 | 5 | 3.5 | http://www.fao.org/agriculture/dairy-gateway/milk-and-milk-products/milk-composition/en/#.VOuGNHa2HsA | |
| per 100 grams of milk | 87.8 | 3.2 | 4.8 | 3.9 | http://en.wikipedia.org/wiki/Milk |
| g per 100ml | 3.3 (casein 2.8) | 4.8 | 3.7 | http://www.britannica.com/EBchecked/topic/327330/lactation/76155/Composition-and-properties-of-milk | |
| percent | 86.5 | 3.5 | 4.8 | 4.5 | http://biotechlearn.org.nz/focus_stories/transgenic_cows/images/composition_of_cow_s_milk |
| milk composition (%) | 2.95 | 4.78 | 4.62 | http://www.ameriflax.com/userfiles/image/tr58.pdf | |
| per liter | 32 | 53 | 33 | http://www.lipidworld.com/content/6/1/25 | |
| average per L | 3.2875 | 4.836 | 4.044 | ||
| per 500ml | 16.4375 | 24.18 | 20.22 |
Salt composition was surveyed as well, the results and calculations are here:
| ' | calcium | phoshpate | magnesium | sodium | potassium | citrate | chlorine | iron | ' |
| form | CaCl2 | TSP | pils | tribasic NaCitrate:2H2O | |||||
| mg/100ml milk | 123 | 95 (Phosphorous (P) ) | 12 | 58 | 141 | 160 | 119 | http://ansci.illinois.edu/static/ansc438/Milkcompsynth/milkcomp_minerals.html | |
| mg/100ml milk | 125 | 96 | 12 | 58 | 138 | http://babcock.wisc.edu/sites/default/files/de/en/de_19.en.pdf | |||
| mg/100ml milk | 125 | 12 | 48 | 138 | 0.09 | http://www.britannica.com/EBchecked/topic/327330/lactation/76155/Composition-and-properties-of-milk | |||
| mg/100ml milk | 110 | 10 | http://www.lipidworld.com/content/6/1/25 | ||||||
| Average mg/100ml | 120.75 | 96 | 11.5 | 54.67 | 139 | 160 | 119 | 0.09 | |
| target molar mass g/M | 40.078 | 24.305 | 189 | ||||||
| concentration by weight in current form (g target/g mixture) | 1 | 0.364431487 | |||||||
| actual form molar mass g/M | 110.98 | 24.305 | 294.1 | ||||||
| target molarity | 0.03013 | 0.00473 | 0.00847 | ||||||
| molarity of saturated solution | ~8M | 2mM as oxide, 1.2mM as gluconate | 356mM |
The BodyTech micellar casein powder lists in its nutritional composition a quantity of calcium that would be equivalent to about 20mM if the micellar casein were added to achieve a final protein concentration of 32.8g/L.
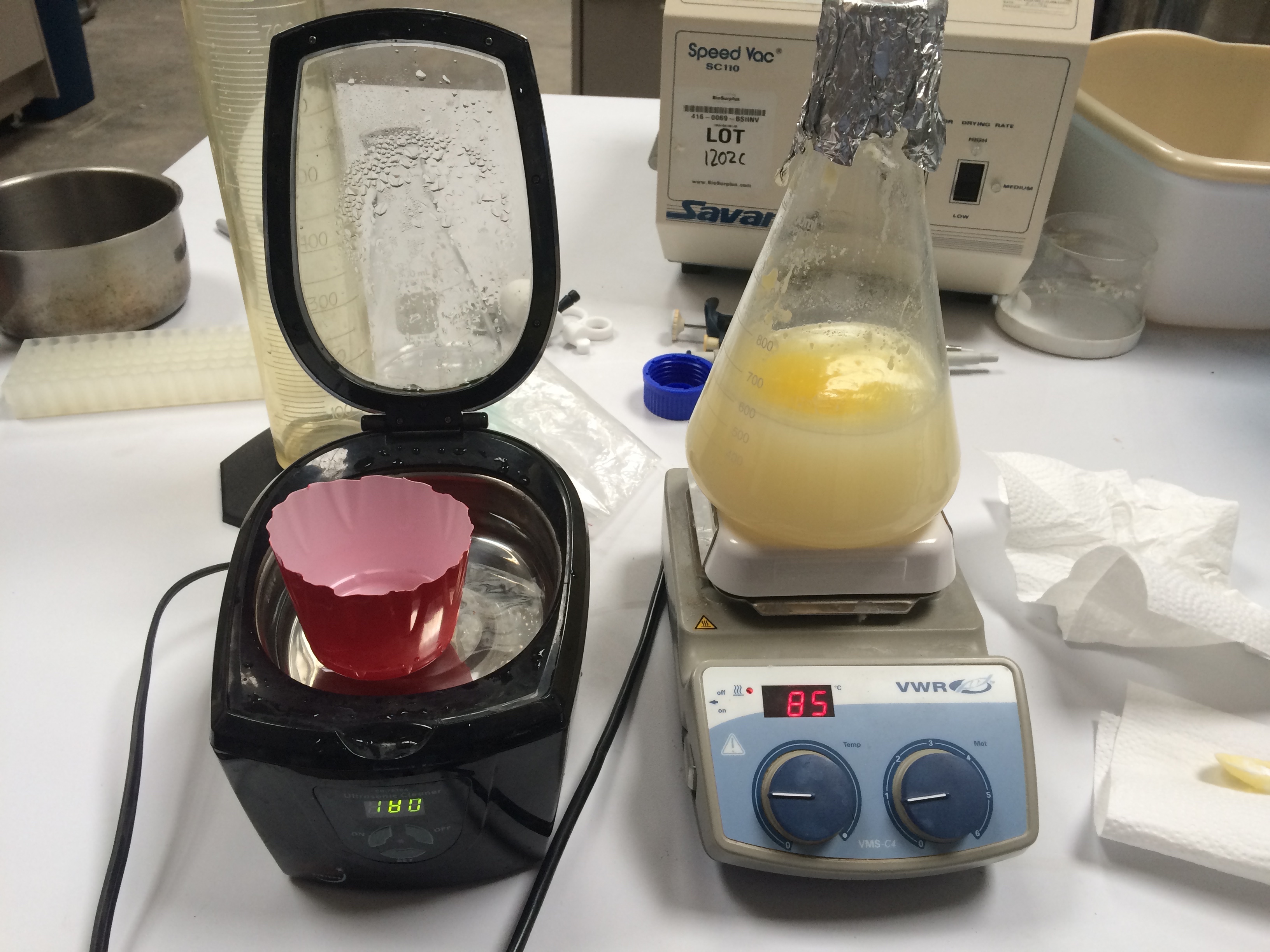
Using this data, re-milk was created with casein powder, lactose, ghee, citrate and a small amount of tri sodium phosphate added as pH buffer. (note: this pHing was in an attempt to match the procedure from Experiment 2, where the pH was adjusted up to 7.5. When attempted production of cheese curds subsequently failed, it was noted that the commonly reported pH of milk is 6.5. As of March 12, why the curd production failed is still being determined, The pH of the re-milk is still suspected, however other factors such as the presence/concentration of divalent cations such as Mg 2+ and Ca 2+ are also being considered ) 500mL of distilled water was placed in an Erlenmeyer flask along with a magnetic stir rod and warmed to 37.5 C on a heated stir plate. The heated stir plate was set to a mixing speed of approximately "0.8", and a temperature setting of around 85, though, the temperature was occasionally varied to achieve a solution temperature between 35 and 38 C (as measured by the Cen-Tech infared thermometer on the part of the liquid surface not covered by liquid ghee.) Citrate was initially added to a achieve a final citrate concentration of 8.47 mM. Casein powder was added to the 500mL water at a rate of 2-5 grams once every 15min, with the full weight of 18.8 g casein ultimately added over 45 minutes. Ghee was added at a rate of 2-5g every 15 minutes, ultimately adding 20.22g over 45 minutes. Lactose was added at a rate of 10-2g every 15 minutes, ultimately adding 24g over 45 minutes. (note: lactose occasionally caused the stir plate motor to stall, requiring the heated stir plate to be re-started) The lactose, ghee and casein were added in a staggered fashion, such that the powdered casein or lactose did not land on a big chunk of solid ghee.
After all ingredients were added the pH of the mixture was found to be approximately 6.8 (using a swimming pool test kit which employed a phenol red indicator and a set of liquid standards. 7µL of phenol red indicator was added to 1 ml of the re-milk mixture.) pH was adjusted to 7.7 by adding two 1mL portions of a saturated (500mM) tri-sodium phosphate (TSP) solution.
This mixture was placed in a fridge over night at approximately 1 degree C. Upon removing from the fridge, a solid layer of ghee was found at the top of the liquid, but no solid precipitate could be found. The mixture was reheated first in a warm water bath, then on the heated stir plate (speed = 1, temp = 85) until the temperature at the liquid surface was measured at approximately 35 C.
Sonication The re-heated re-milk mixture was shaken to attempt to distribute the oil layer, then approximately 125 mL of the re-milk was poured into a cut off (to ~ 3") dixie cup and placed in a the sonicator. Warm water was added to the sonicator bath to bring it to the same level as the re-milk in the dixie cup. The sonicator was then run for 32 minutes. The sonication did cause heating of the water, and at 24 minutes the water bath temperature was found to be 42 C; cool water was added to bring the temperature down to 34 C.
Results of milk re-assembly
Initial mixture of ingredients produced a translucent yellowish solution with a layer of
Sonication made a significant change in the re-milk's appearance, giving it a nice opaque white, much more milk-like quality. It is difficult to tell if the sonication had a significant lasting or permanent effect on the amount of liquid ghee in a stable emulsion -- persisting not in the non-polar layer on the top, but in solution with the re-milk/lactose layer on the botton. Comparing the photographs at 15 and 32 minutes, it appears the sonication at least transiently drove some of the oil layer into solution.
The cheese making recipe used here attempts to replicate the instructions found a the ["Gouda, A Cheese from the North Country"] page of the cheesemaking.com website.
Approximately 125mL of re-milk was first warmed in a water bath to 31 degrees C. Once the temperature was reached, 50mg of mesophillic, c101 culture was added and allowed to incubate for 30 minutes. During incubation, temperature was found to vary between 30 and 36 degrees. The pH at the end of the incubation was measured and found to be 7.5. Addition rennet was performed by first diluting 14µL of the double strength vegetable rennet to 280µL, then mixing it into the re-milk for 2 minutes. As to the instructions, curds should have formed by 40 minuts, however, at 40 minutes no noticeable curd formation or thickening of the re-milk could be detected. The pH was then adjusted down to about 6.8 with addition of 1:100 solution of HCl, and the culture and rennet were again added, following the above described procedure. After the 30 minute incubation and 40 minute rennet reaction, again no noticeable curd formation could be detected.
Testing of the culture and rennet
The culture and rennet was tested on real whole milk from Trader Joes. In short, 125mL of milk was heated to 30 degrees C. The pH was tested and was found to be more acidic than the pH test kit's lowest range (see photo). 50mg of mesophilic culture was then added, allowed to absorb in for 2 minutes, then mixed into the warm milk. After 30 minutes, the pH was measured again, showing no apparent change. The vegetable rennet was then added and mixed in for 2 minutes. This mixture was then allowed to sit at 30 degrees C for 40 minutes. At then end of this time, curd was observed -- the milk had congealed to a consistency similar to Greek yogurt. This was taken as evidence that the rennet was still viable. The curd was placed in the fridge and cheese
Discussion
Possible factors preventing curd formation:
- the rennet may have been accidentally frozen while in storage.
- pH not in the correct range for the catalytic activity of the rennet
- poor accuracy of the pH measurement device may have belied the actual pH of the re-milk.
- Salt composition (Ca2+, phosphate, citrate, Mg2+)
- buffering by the added phosphate may have prevented acidification by the culture
- mal-formed casein micelles may have made the cleavage site in the kappa casein un-accessible to the rennet.
Next steps:
- Try using the rennet and culture on real milk.
This may indicate if the rennet & culture are still viable. Unwanted temperature fluctuations may have damaged one of these two ingredients.
- Try not raising the pH.
- Try changing the composition of salts: Ca 2+ increase, Mg 2+ increase.
After a brief search, several sources have been found which indicate the clotting process has some Ca2+ dependence. (http://www1.lsbu.ac.uk/water/enztech/proteases.html, http://www.tandfonline.com/doi/pdf/10.1080/00021369.1987.10868318). Although the casein powder is known to have some calcium, it is possible this calcium is tightly bound (by the casein, phosphate or in complex with casein as phosphate-casein nano clusters) making it unavailable to the rennet/chymosin.